A Comparison between Mechanisms of Multi-Alternative Perceptual Decision Making: Ability to Explain Human Behavior, Predictions for Neurophysiology, and Relationship with Decision Theory
- PMID: 21152262
- PMCID: PMC2999395
- DOI: 10.3389/fnins.2010.00184
A Comparison between Mechanisms of Multi-Alternative Perceptual Decision Making: Ability to Explain Human Behavior, Predictions for Neurophysiology, and Relationship with Decision Theory
Abstract
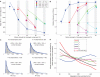
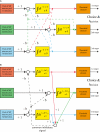
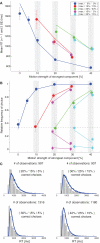
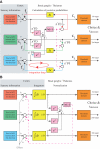
While there seems to be relatively wide agreement about perceptual decision making relying on integration-to-threshold mechanisms, proposed models differ in a variety of details. This study compares a range of mechanisms for multi-alternative perceptual decision making, including integration with and without leakage, feedforward and feedback inhibition for mediating the competition between integrators, as well as linear and non-linear mechanisms for combining signals across alternatives. It is shown that a number of mechanisms make very similar predictions for the decision behavior and are therefore able to explain previously published data from a multi-alternative perceptual decision task. However, it is also demonstrated that the mechanisms differ in their internal dynamics and therefore make different predictions for neuorphysiological experiments. The study further addresses the relationship of these mechanisms with decision theory and statistical testing and analyzes their optimality.
Keywords: behavior; decision theory; mathematical models; multiple alternatives; neurophysiology; perceptual decisions.
Figures



References
-
- Bogacz R. (2009). “Optimal decision-making theories,” in Handbook of Reward and Decision Making, ed. Dreher J.-C. T. (Oxford: Academic Press; ), 375–397
LinkOut - more resources
Full Text Sources

