Microfluidic tools for cell biological research
- PMID: 21152269
- PMCID: PMC2998071
- DOI: 10.1016/j.nantod.2009.12.001
Microfluidic tools for cell biological research
Abstract
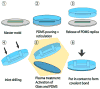
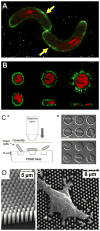
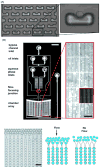
Microfluidic technology is creating powerful tools for cell biologists to control the complete cellular microenvironment, leading to new questions and new discoveries. We review here the basic concepts and methodologies in designing microfluidic devices, and their diverse cell biological applications.
Figures







References
-
- Campbell CJ, Grzybowski BA. Philos Trans A Math Phys Eng Sci. 2004;362:1069. - PubMed
-
- Stroock AD, Dertinger SK, Ajdari A, Mezic I, Stone HA, Whitesides GM. Science. 2002;295:647. - PubMed
-
- Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal Chem. 1998;70:4974. - PubMed
-
- McDonald JC, Whitesides GM. Acc Chem Res. 2002;35:491. - PubMed
-
- Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Science. 2000;288:113. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
