Development and application of versatile bis-hydroxamic acids for catalytic asymmetric oxidation
- PMID: 21152351
- PMCID: PMC2998186
- DOI: 10.1016/j.tet.2007.03.071
Development and application of versatile bis-hydroxamic acids for catalytic asymmetric oxidation
Abstract
In this article, we describe the development and preliminary results of our new designed C(2)-symmetric bis-hydroxamic acid (BHA) ligands and the application of the new ligands for vanadium-catalyzed asymmetric epoxidation of allylic alcohols as well as homoallylic alcohols. From this success we demonstrate the versatile nature of BHA in the molybdenum catalyzed asymmetric oxidation of unfunctionalized olefins and sulfides.
Figures
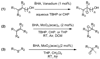
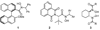



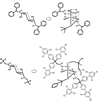

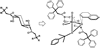
References
-
-
For recent reviews, see Katsuki T. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 2. Berlin: Springer; 1999. p. 621. Katsuki T, Ojima I. Org. React. 1996;48:1. Keith JM, Larrow JF, Jacobsen EN. Adv. Synth. Catal. 2001;343:5. Adam W, Saha-Möller CR, Ganeshpure PA. Chem. Rev. 2001;101:3499. Adam W, Malisch W, Roschmann KJ, Möller CR, Schenk WA. J. Organomet. Chem. 2002;661:3. Lattanzi A, Scettri A. J. Organomet. Chem. 2006;691:2072. Shi Y. Acc. Chem. Res. 2004;37:488. Shi Y, Frohn M. Synthesis. 2000;14:1979.
-
-
- Katsuki T, Sharpless KB. J. Am. Chem. Soc. 1980;102:5974.
- Rossiter BE, Sharpless KB. J. Org. Chem. 1984;49:3707.
- Gao Y, Hanson RM, Klunder JM, Ko SY, Masamune H, Sharpless KB. J. Am. Chem. Soc. 1987;109:5765.
-
-
For recent reports on chiral vanadium catalyst for epoxidation, see Bryliakov KP, Talsi EP. Kinetics and Catalysis (Translation of Kinetika i Kataliz) 2003;44:334. Bolm C, Kuhn T. Synlett. 2000:899. Traber B, Jung YG, Park TK, Hong JI. Bull. Korean Chem. Soc. 2001;22:547. Wu HL, Uang BJ. Tetrahedron: Asymmetry. 2002;13:2625. Bourhani Z, Malkov AV. Chem. Commun. 2005:4592. Lattanzi A, Piccirillo S, Scettri A. Eur. J. Org. Chem. 2005:1669.
-
-
-
For recent reports on achiral metal catalyst and chiral oxidant see Adam W, Alsters PL, Neumann R, Saha-Möller CR, Seebach D, Beck AK, Zhang R. J. Org. Chem. 2003;68:8222. Adam W, Beck AK, Pichota A, Saha-Möller CR, Seebach D, Vogl N, Zhang R. Tetrahedron: Asymmetry. 2003;14:1355. Adam W, Alsters PL, Neumann R, Saha-Möller CR, Seebach D, Zhang R. Org. Lett. 2003;5:725.
-
-
-
For recent reviews on vanadium see, Hirao T. Chem. Rev. 1997;97:2707. Bolm C. Coordination Chemistry Reviews. 2003;237:245.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
