IGF-IR internalizes with Caveolin-1 and PTRF/Cavin in HaCat cells
- PMID: 21152401
- PMCID: PMC2994771
- DOI: 10.1371/journal.pone.0014157
IGF-IR internalizes with Caveolin-1 and PTRF/Cavin in HaCat cells
Abstract
Background: Insulin-like growth factor-I receptor (IGF-IR) is a tyrosine kinase receptor (RTK) associated with caveolae, invaginations of the plasma membrane that regulate vesicular transport, endocytosis and intracellular signaling. IGF-IR internalization represents a key mechanism of down-modulation of receptors number on plasma membrane. IGF-IR interacts directly with Caveolin-1 (Cav-1), the most relevant protein of caveolae. Recently it has been demonstrated that the Polymerase I and Transcript Release Factor I (PTRF/Cavin) is required for caveolae biogenesis and function. The role of Cav-1 and PTRF/Cavin in IGF-IR internalization is still to be clarified.
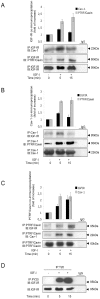
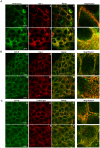
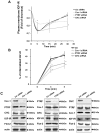
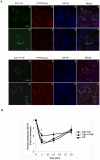
Methodology/principal findings: We have investigated the interaction of IGF-IR with Cav-1 and PTRF/Cavin in the presence of IGF1in human Hacat cells. We show that IGF-IR internalization triggers Cav-1 and PTRF/Cavin translocation from plasma membrane to cytosol and increases IGF-IR interaction with these proteins. In fact, Cav-1 and PTRF/Cavin co-immunoprecipitate with IGF-IR during receptor internalization. We found a different time course of co-immunoprecipitation between IGF-IR and Cav-1 compared to IGF-IR and PTRF/Cavin. Cav-1 and PTRF/Cavin silencing by siRNA differently affect surface IGF-IR levels following IGF1 treatment: Cav-1 and PTRF/Cavin silencing significantly affect IGF-IR rate of internalization, while PTRF/Cavin silencing also decreases IGF-IR plasma membrane recovery. Since Cav-1 phosphorylation could have a role in IGF-IR internalization, the mutant Cav-1Y14F lacking Tyr14 was transfected. Cav-1Y14F transfected cells showed a reduced internalization of IGF-IR compared with cells expressing wild type Cav-1. Receptor internalization was not impaired by Clathrin silencing. These findings support a critical role of caveolae in IGF-IR intracellular traveling.
Conclusions/significance: These data indicate that Caveolae play a role in IGF-IR internalization. Based on these findings, Cav-1 and PTRF/Cavin could represent two relevant and distinct targets to modulate IGF-IR function.
Conflict of interest statement
Figures

References
-
- Werner H, Bruchim I. The insulin-like growth factor-I receptor as an oncogene. Arch Physiol Biochem. 2009;115:58–71. - PubMed
-
- Salani B, Briatore L, Garibaldi S, Cordera R, Maggi D. Caveolin-1 down regulation inhibits IGF-IR signal transduction in H9C2 rat cardio myoblasts. Endocrinology 2007 - PubMed
-
- Matthews LC, Taggart MJ, Westwood M. Effect of cholesterol depletion on mitogenesis and survival: the role of caveolar and noncaveolar domains in insulin-like growth factor-mediated cellular function. Endocrinology. 2005;146:5463–5473. - PubMed
-
- Baserga R. The IGF-I receptor in cancer research. Exp Cell Res. 1999;253:1–6. - PubMed
-
- Zapf A, Hsu D, Olefsky JM. Comparison of the intracellular itineraries of insulin-like growth factor-I and insulin and their receptors in Rat-1 fibroblasts. Endocrinology. 1994;134:2445–2452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

