Independent interactions of phosphorylated β-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions
- PMID: 21152425
- PMCID: PMC2994709
- DOI: 10.1371/journal.pone.0014127
Independent interactions of phosphorylated β-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions
Abstract
Background: The APC tumour suppressor functions in several cellular processes including the regulation of β-catenin in Wnt signalling and in cell adhesion and migration.
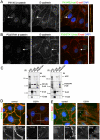
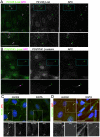
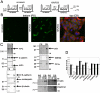
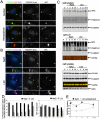
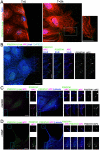
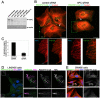
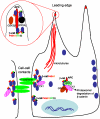
Findings: In this study, we establish that in epithelial cells N-terminally phosphorylated β-catenin specifically localises to several subcellular sites including cell-cell contacts and the ends of cell protrusions. N-terminally phosphorylated β-catenin associates with E-cadherin at adherens junctions and with APC in cell protrusions. We isolated APC-rich protrusions from stimulated cells and detected β-catenin, GSK3β and CK1α, but not axin. The APC/phospho-β-catenin complex in cell protrusions appears to be distinct from the APC/axin/β-catenin destruction complex. GSK3β phosphorylates the APC-associated population of β-catenin, but not the cell junction population. β-catenin associated with APC is rapidly phosphorylated and dephosphorylated. HGF and wound-induced cell migration promote the localised accumulation of APC and phosphorylated β-catenin at the leading edge of migrating cells. APC siRNA and analysis of colon cancer cell lines show that functional APC is required for localised phospho-β-catenin accumulation in cell protrusions.
Conclusions: We conclude that N-terminal phosphorylation of β-catenin does not necessarily lead to its degradation but instead marks distinct functions, such as cell migration and/or adhesion processes. Localised regulation of APC-phospho-β-catenin complexes may contribute to the tumour suppressor activity of APC.
Conflict of interest statement
Figures

Similar articles
-
Adenomatous polyposis coli regulates endothelial cell migration independent of roles in beta-catenin signaling and cell-cell adhesion.Mol Biol Cell. 2010 Aug 1;21(15):2611-23. doi: 10.1091/mbc.e10-03-0235. Epub 2010 Jun 2. Mol Biol Cell. 2010. PMID: 20519433 Free PMC article.
-
Reversible modification of adenomatous polyposis coli (APC) with K63-linked polyubiquitin regulates the assembly and activity of the β-catenin destruction complex.J Biol Chem. 2012 Aug 17;287(34):28552-63. doi: 10.1074/jbc.M112.387878. Epub 2012 Jul 3. J Biol Chem. 2012. PMID: 22761442 Free PMC article.
-
different Roles for the axin interactions with the SAMP versus the second twenty amino acid repeat of adenomatous polyposis coli.PLoS One. 2014 Apr 10;9(4):e94413. doi: 10.1371/journal.pone.0094413. eCollection 2014. PLoS One. 2014. PMID: 24722208 Free PMC article.
-
APC shuttling to the membrane, nucleus and beyond.Trends Cell Biol. 2008 Dec;18(12):587-96. doi: 10.1016/j.tcb.2008.09.002. Epub 2008 Oct 9. Trends Cell Biol. 2008. PMID: 18848448 Review.
-
Adenomatous polyposis coli proteins and cell adhesion.Curr Opin Cell Biol. 2004 Oct;16(5):528-35. doi: 10.1016/j.ceb.2004.08.001. Curr Opin Cell Biol. 2004. PMID: 15363803 Review.
Cited by
-
Adenomatous Polyposis Coli (APC) in cell migration.Eur J Cell Biol. 2022 Jun-Aug;101(3):151228. doi: 10.1016/j.ejcb.2022.151228. Epub 2022 Apr 22. Eur J Cell Biol. 2022. PMID: 35483122 Free PMC article. Review.
-
GTW inhibits the Epithelial to Mesenchymal Transition of Epithelial Ovarian Cancer via ILK/AKT/GSK3β/Slug Signalling Pathway.J Cancer. 2021 Jan 1;12(5):1386-1397. doi: 10.7150/jca.52418. eCollection 2021. J Cancer. 2021. PMID: 33531984 Free PMC article.
-
Mechanism of APC truncation involved in colorectal cancer tumorigenesis (Review).Oncol Lett. 2024 Oct 15;29(1):2. doi: 10.3892/ol.2024.14748. eCollection 2025 Jan. Oncol Lett. 2024. PMID: 39526304 Free PMC article. Review.
-
APC/β-catenin-rich complexes at membrane protrusions regulate mammary tumor cell migration and mesenchymal morphology.BMC Cancer. 2013 Jan 9;13:12. doi: 10.1186/1471-2407-13-12. BMC Cancer. 2013. PMID: 23302090 Free PMC article.
-
Beta-catenin phosphorylated at threonine 120 antagonizes generation of active beta-catenin by spatial localization in trans-Golgi network.PLoS One. 2012;7(4):e33830. doi: 10.1371/journal.pone.0033830. Epub 2012 Apr 12. PLoS One. 2012. PMID: 22511927 Free PMC article.
References
-
- Cottrell S, Bicknell D, Kaklamanis L, Bodmer WF. Molecular analysis of APC mutations in familial adenomatous polyposis and sporadic colon carcinomas. Lancet. 1992;340:626–630. - PubMed
-
- Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. - PubMed
-
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

