Biased signaling of the angiotensin II type 1 receptor can be mediated through distinct mechanisms
- PMID: 21152433
- PMCID: PMC2994726
- DOI: 10.1371/journal.pone.0014135
Biased signaling of the angiotensin II type 1 receptor can be mediated through distinct mechanisms
Abstract
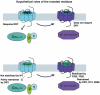
Background: Seven transmembrane receptors (7TMRs) can adopt different active conformations facilitating a selective activation of either G protein or β-arrestin-dependent signaling pathways. This represents an opportunity for development of novel therapeutics targeting selective biological effects of a given receptor. Several studies on pathway separation have been performed, many of these on the Angiotensin II type 1 receptor (AT1R). It has been shown that certain ligands or mutations facilitate internalization and/or recruitment of β-arrestins without activation of G proteins. However, the underlying molecular mechanisms remain largely unresolved. For instance, it is unclear whether such selective G protein-uncoupling is caused by a lack of ability to interact with G proteins or rather by an increased ability of the receptor to recruit β-arrestins. Since uncoupling of G proteins by increased ability to recruit β-arrestins could lead to different cellular or in vivo outcomes than lack of ability to interact with G proteins, it is essential to distinguish between these two mechanisms.
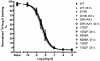
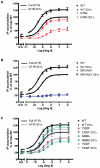
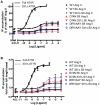
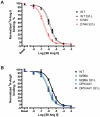
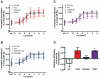
Methodology/principal findings: We studied five AT1R mutants previously published to display pathway separation: D74N, DRY/AAY, Y292F, N298A, and Y302F (Ballesteros-Weinstein numbering: 2.50, 3.49-3.51, 7.43, 7.49, and 7.53). We find that D74N, DRY/AAY, and N298A mutants are more prone to β-arrestin recruitment than WT. In contrast, receptor mutants Y292F and Y302F showed impaired ability to recruit β-arrestin in response to Sar1-Ile4-Ile8 (SII) Ang II, a ligand solely activating the β-arrestin pathway.
Conclusions/significance: Our analysis reveals that the underlying conformations induced by these AT1R mutants most likely represent principally different mechanisms of uncoupling the G protein, which for some mutants may be due to their increased ability to recruit β-arrestin2. Hereby, these findings have important implications for drug discovery and 7TMR biology and illustrate the necessity of uncovering the exact molecular determinants for G protein-coupling and β-arrestin recruitment, respectively.
Conflict of interest statement
Figures


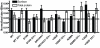


References
-
- Aplin M, Christensen GL, Hansen JL. Pharmacologic perspectives of functional selectivity by the angiotensin II type 1 receptor. Trends Cardiovasc Med. 2008;18:305–312. - PubMed
-
- Nygaard R, Frimurer TM, Holst B, Rosenkilde MM, Schwartz TW. Ligand binding and micro-switches in 7TM receptor structures. Trends Pharmacol Sci. 2009;30:249–259. - PubMed
-
- Ballesteros JA, Shi L, Javitch JA. Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors. Mol Pharmacol. 2001;60:1–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

