A comparative study on the ability of two implicit solvent lipid models to predict transmembrane helix tilt angles
- PMID: 21152910
- PMCID: PMC3030950
- DOI: 10.1007/s00232-010-9325-7
A comparative study on the ability of two implicit solvent lipid models to predict transmembrane helix tilt angles
Abstract
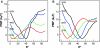
Free-energy profiles describing the relative orientation of membrane proteins along predefined coordinates can be efficiently calculated by means of umbrella simulations. Such simulations generate reliable orientational distributions but are difficult to converge because of the very long equilibration times of the solvent and the lipid bilayer in explicit representation. Two implicit lipid membrane models are here applied in combination with the umbrella sampling strategy to the simulation of the transmembrane (TM) helical segment from virus protein U (Vpu). The models are used to study both orientation and energetics of this α-helical peptide as a function of hydrophobic mismatch. We observe that increasing the degree of positive hydrophobic mismatch increased the tilt angle of Vpu. These findings agree well with experimental data and as such validate the solvation models used in this study.
Figures

Similar articles
-
Revisiting hydrophobic mismatch with free energy simulation studies of transmembrane helix tilt and rotation.Biophys J. 2010 Jul 7;99(1):175-83. doi: 10.1016/j.bpj.2010.04.015. Biophys J. 2010. PMID: 20655845 Free PMC article.
-
Implicit membrane treatment of buried charged groups: application to peptide translocation across lipid bilayers.Biochim Biophys Acta. 2014 Sep;1838(9):2149-59. doi: 10.1016/j.bbamem.2014.01.015. Epub 2014 Feb 10. Biochim Biophys Acta. 2014. PMID: 24525075 Free PMC article.
-
Analyzing the effects of hydrophobic mismatch on transmembrane α-helices using tryptophan fluorescence spectroscopy.Methods Mol Biol. 2013;1063:95-116. doi: 10.1007/978-1-62703-583-5_5. Methods Mol Biol. 2013. PMID: 23975773
-
Orientation and dynamics of transmembrane peptides: the power of simple models.Eur Biophys J. 2010 Mar;39(4):609-21. doi: 10.1007/s00249-009-0567-1. Epub 2009 Dec 18. Eur Biophys J. 2010. PMID: 20020122 Free PMC article. Review.
-
Implicit solvent simulation models for biomembranes.Eur Biophys J. 2006 Jan;35(2):104-24. doi: 10.1007/s00249-005-0013-y. Epub 2005 Sep 27. Eur Biophys J. 2006. PMID: 16187129 Review.
Cited by
-
Prediction, refinement, and persistency of transmembrane helix dimers in lipid bilayers using implicit and explicit solvent/lipid representations: microsecond molecular dynamics simulations of ErbB1/B2 and EphA1.Proteins. 2013 Mar;81(3):365-76. doi: 10.1002/prot.24192. Epub 2012 Nov 5. Proteins. 2013. PMID: 23042146 Free PMC article.
References
-
- Bennett CH. Efficient estimation of free-energy differences from Monte-Carlo data. J Comput Phys. 1976;22:245–268. doi: 10.1016/0021-9991(76)90078-4. - DOI
-
- Brannigan G, Lin LCL, Brown FLH. Implicit solvent simulation models for biomembranes. Eur Biophys J Biophys Lett. 2006;35:104–124. - PubMed
-
- de Planque MRR, Bonev BB, Demmers JAA, Greathouse DV, Koeppe RE, II, Separovic F, Watts A, Killian JA. Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide–lipid interactions. Biochemistry. 2003;42:5341–5348. doi: 10.1021/bi027000r. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

