Methylxanthine treatment for apnoea in preterm infants
- PMID: 21154343
- PMCID: PMC11751766
- DOI: 10.1002/14651858.CD000140.pub2
Methylxanthine treatment for apnoea in preterm infants
Abstract
Background: Recurrent apnoea is common in preterm infants, particularly at very early gestational ages. These episodes of ineffective breathing can lead to hypoxaemia and bradycardia that may be severe enough to require the use of positive pressure ventilation. Methylxanthines (such as caffeine, theophylline or aminophylline) have been used to stimulate breathing and reduce apnoea and its consequences.
Objectives: To determine the effects of methylxanthine treatment on the incidence of apnoea and the use of intermittent positive pressure ventilation (IPPV) and other clinically important outcomes in preterm infants with recurrent apnoea.
Search strategy: Searches were made of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2010), the Oxford Database of Perinatal Trials, MEDLINE (1966 to June 2010), EMBASE (1982 to June 2010), previous reviews including cross references, abstracts, conferences and symposia proceedings, expert informants, journal hand searching mainly in the English language.
Selection criteria: All trials utilizing random or quasi-random patient allocation in which methylxanthine (theophylline, caffeine or aminophylline) as treatment for apnoea was compared with placebo or no treatment for apnoea in preterm infants were included.
Data collection and analysis: Methodological quality was assessed independently by the review authors. Data were extracted independently by the review authors. Analysis was done in accordance with the recommendations of the Cochrane Neonatal Review Group.
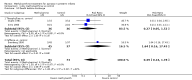
Main results: Six trials reported on the effect of methylxanthine in the treatment of apnoea (three trials of theophylline and three trials of caffeine). Five trials that enrolled a total of 192 preterm infants with apnoea evaluated short term outcomes; in these studies, methylxanthine therapy led to a reduction in apnoea and use of IPPV in the first two to seven days. The post-hoc analysis of the large CAP Trial comparing caffeine to control in a subgroup of infants being treated for apnoea reported significantly reduced rates of PDA ligation; postmenstrual age at last oxygen treatment, last endotracheal tube use, last positive pressure ventilation; and reduced chronic lung disease at 36 weeks.
Authors' conclusions: Methylxanthine is effective in reducing the number of apnoeic attacks and the use of mechanical ventilation in the two to seven days after starting treatment. Caffeine is also associated with better longer term outcomes. In view of its lower toxicity, caffeine would be the preferred drug for the treatment of apnoea.
Conflict of interest statement
None
Figures











Update of
-
Methylxanthine treatment for apnea in preterm infants.Cochrane Database Syst Rev. 2001;(3):CD000140. doi: 10.1002/14651858.CD000140. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2010 Dec 08;(12):CD000140. doi: 10.1002/14651858.CD000140.pub2. PMID: 11686952 Updated.
Comment in
-
In preterm infants with recurrent apnoea, methylxanthine reduces the number of episodes and the use of mechanical ventilation in the short term; caffeine is also associated with improved longer term outcomes.Evid Based Med. 2011 Aug;16(4):120-1. doi: 10.1136/ebm1207. Epub 2011 Mar 29. Evid Based Med. 2011. PMID: 21447523 No abstract available.
References
References to studies included in this review
CAP Trial 2006 {published data only}
-
- Davis PG, Schmidt B, Roberts RS, Doyle LW, Asztalos E, Haslam R, Sinha S, Tin W, Caffeine for Apnea of Prematurity Trial Group. Caffeine for Apnea of Prematurity trial: benefits may vary in subgroups. Journal of Pediatrics 2010;156(3):382‐7. - PubMed
-
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W, Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. New England Journal of Medicine 2006;354(20):2112‐21. - PubMed
-
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W, Caffeine for Apnea of Prematurity Trial Group. Long‐term effects of caffeine therapy for apnea of prematurity. New England Journal of Medicine 2007;357(19):1893‐902. - PubMed
Erenberg 2000 {published data only}
-
- Erenberg A, Leff R, Wynne B. Results of the first double blind placebo (PL) controlled study of caffeine citrate (CC) for the treatment of apnea of prematurity (AOP). Pediatric Research 1998;43:172A. - PubMed
-
- Erenberg A, Leff RD, Haack DG, Mosdell KW, Hicks GM, Wynne BA. Caffeine citrate for the treatment of apnea of prematurity: a double‐blind placebo‐controlled study. Pharmacotherapy 2000;20(6):644‐52. - PubMed
Gupta 1981 {published and unpublished data}
-
- Gupta JM, Mercer HP, Koo WWK. Theophylline in treatment of apnoea of prematurity. Australian Paediatric Journal 1981;17(4):290‐1. - PubMed
Murat 1981 {published data only}
-
- Murat I, Moriette G, Blin MC, Couchard M, Flouvat B, Gamarra E, et al. The efficacy of caffeine in the treatment of recurrent idiopathic apnea in premature infants. Journal of Pediatrics 1981;99(6):984‐99. - PubMed
Peliowski 1990 {published data only}
-
- Peliowski A, Finer NN. A blinded, randomized, placebo‐controlled trial to compare theophylline and doxapram for the treatment of apnea of prematurity. Journal of Pediatrics 1990;116(4):648‐53. - PubMed
Sims 1985 {published data only}
-
- Sims ME, Yau G, Rambhatla S, Cabal L, Wu PYK. Limitations of theophylline in the treatment of apnea of prematurity. American Journal of Diseases of Children 1985;139(6):567‐70. - PubMed
References to studies excluded from this review
Charles 2008 {published data only}
-
- Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A. Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Therapeutic Drug Monitoring 2008;30(6):709‐16. - PubMed
Hochwald 2002 {published data only}
-
- Hochwald C, Kennedy K, Chang J, Moya F. A randomized, controlled, double‐blind trial comparing two loading doses of aminophylline. Journal of Perinatology 2002;22(4):275‐8. - PubMed
Additional references
AAP 2003
-
- Committee on Fetus and Newborn. American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 2003;111(4 Pt 1):914‐22. - PubMed
Blanchard 1992
-
- Blanchard PW, Aranda JV. Pharmacotherapy of respiratory control disorders. In: Beckerman RC, Brouillette RT, Hunt CE editor(s). Respiratory Control Disorders in Infants and Children. Baltimore: Williams & Wilkins, 1992:352‐370.
Comer 2001
-
- Comer AM, Perry CM, Figgitt DP. Caffeine citrate: a review of its use in apnoea of prematurity. Paediatric Drugs 2001;3(1):61‐79. - PubMed
Davis 2000
-
- Davis PG, Doyle LW, Rickards AL, Kelly EA, Ford GW, Davis NM, Callanan C. Methylxanthines and sensorineural outcome at 14 years in children < 1501 g birthweight. Journal of Paediatrics and Child Health 2000;36(1):47‐50. - PubMed
Henderson‐Smart 2006
Henderson‐Smart 2008
Henderson‐Smart 2004
-
- Henderson‐Smart DJ. Recurrent apnoea. Evidence Based Pediatrics. Oxford: Blackwell, 2004.
Henderson‐Smart 2010
Martin 1998
-
- Martin RJ, Fanaroff AA. Neonatal apnea, bradycardia, or desaturation: does it Matter?. Journal of Pediatrics 1998;132(5):758‐759. - PubMed
Samuels 1992
-
- Samuels MP, Southall DP. Recurrent apnea. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:385‐97.
Schmidt 1999
-
- Schmidt B. Methylxanthine therapy in premature infants: sound practice, disaster or fruitless byway?. Journal of Pediatrics 1999;135(4):526‐8. - PubMed
References to other published versions of this review
Henderson‐Smart 2004a
-
- Henderson‐Smart DJ, Steer P. Methylxanthine for apnea in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD000140] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

