The legumain protease-activated auristatin prodrugs suppress tumor growth and metastasis without toxicity
- PMID: 21154805
- PMCID: PMC3549592
- DOI: 10.1002/cmdc.201000478
The legumain protease-activated auristatin prodrugs suppress tumor growth and metastasis without toxicity
Figures
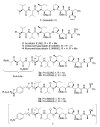



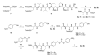
References
-
- Pettit GR, Kamano Y, Herald CL, Tuinman AA, Boettner FE, Kizu H, Schmidt JM, Baczynskyj L, Tomer KB, Bontems RJ. J. Am. Chem. Soc. 1987;109:6883.
-
- Pettit GR, Srirangam JK, Barkoczy J, Williams MD, Boyd MR, Hamel E, Pettit RK, Hogan F, Bai R, Chapuis JC, McAllister SC, Schmidt JM. Anti-Cancer Drug Des. 1998;13:243. - PubMed
-
- Prokopiou EM, Cooper PA, Pettit GR, Bibby MC, Shnyder SD. Mol. Med. Rep. 2010;3:309. - PubMed
-
- Vaishampayan U, Glode M, Du W, Kraft A, Hudes G, Wright J, Hussain M. Clin. Cancer Res. 2000;6:4205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

