Proteoglycans in cancer biology, tumour microenvironment and angiogenesis
- PMID: 21155971
- PMCID: PMC3633488
- DOI: 10.1111/j.1582-4934.2010.01236.x
Proteoglycans in cancer biology, tumour microenvironment and angiogenesis
Abstract
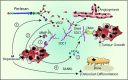
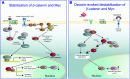
Proteoglycans, key molecular effectors of cell surface and pericellular microenvironments, perform multiple functions in cancer and angiogenesis by virtue of their polyhedric nature and their ability to interact with both ligands and receptors that regulate neoplastic growth and neovascularization. Some proteoglycans such as perlecan, have pro- and anti-angiogenic activities, whereas other proteoglycans, such as syndecans and glypicans, can also directly affect cancer growth by modulating key signalling pathways. The bioactivity of these proteoglycans is further modulated by several classes of enzymes within the tumour microenvironment: (i) sheddases that cleave transmembrane or cell-associated syndecans and glypicans, (ii) various proteinases that cleave the protein core of pericellular proteoglycans and (iii) heparanases and endosulfatases which modify the structure and bioactivity of various heparan sulphate proteoglycans and their bound growth factors. In contrast, some of the small leucine-rich proteoglycans, such as decorin and lumican, act as tumour repressors by physically antagonizing receptor tyrosine kinases including the epidermal growth factor and the Met receptors or integrin receptors thereby evoking anti-survival and pro-apoptotic pathways. In this review we will critically assess the expanding repertoire of molecular interactions attributed to various proteoglycans and will discuss novel proteoglycan functions modulating cancer progression, invasion and metastasis and how these factors regulate the tumour microenvironment.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

References
-
- Adany R, Heimer R, Caterson B, et al. Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem. 1990;265:11389–96. - PubMed
-
- Iozzo RV. Tumor stroma as a regulator of neoplastic behavior. Agonistic and antagonistic elements embedded in the same connective tissue. Lab Invest. 1995;73:157–60. - PubMed
-
- Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nature Rev Mol Cell Biol. 2005;6:646–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

