Efficacy and tolerability of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: sex and age effects and effect size across the day
- PMID: 21156071
- PMCID: PMC3022598
- DOI: 10.1186/1753-2000-4-32
Efficacy and tolerability of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: sex and age effects and effect size across the day
Abstract
Background: Efficacy and safety profiles by sex and age (6-9 vs 10-12 years) and magnitude and duration of effect by effect size overall and across the day of lisdexamfetamine dimesylate (LDX) vs placebo were assessed.
Methods: This study enrolled children (6-12 years) with attention-deficit/hyperactivity disorder (ADHD) in an open-label dose optimization with LDX (30-70 mg/d) followed by a randomized, double-blind, placebo-controlled, 2-way crossover phase. Post hoc analyses assessed interaction between sex or age and treatment and assessed effect sizes for Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) and Permanent Product Measure of Performance (PERMP) scales and ADHD Rating Scale IV measures. No corrections for multiple testing were applied on time points and subgroup statistical comparisons.
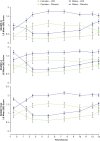
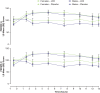
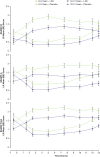
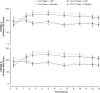
Results: 129 participants enrolled; 117 randomized. Both sexes showed improvement on all assessments at postdose time points; females showed less impairment than males for SKAMP and PERMP scores in treatment and placebo groups at nearly all times. Both age groups improved on all assessments at postdose time points. Children 10-12 years had less impairment in SKAMP ratings than those 6-9 years. Treatment-by-sex interactions were observed at time points for SKAMP-D, SKAMP total, and PERMP scores; no consistent pattern across scales or time points was observed. LDX demonstrated significant improvement vs placebo, by effect size, on SKAMP-D from 1.5-13 hours postdose. The overall LS mean (SE) SKAMP-D effect size was -1.73 (0.18). In the dose-optimization phase, common (≥2%) treatment-emergent adverse events (TEAEs) in males were upper abdominal pain, headache, affect lability, initial insomnia, and insomnia; in females were nausea and decreased weight. During the crossover phase for those taking LDX, higher incidence (≥2% greater) was observed in males for upper abdominal pain and insomnia and in females for nausea and headache. Overall incidence of TEAEs in age groups was similar.
Conclusion: Apparent differences in impairment level between sex and age groups were noted. However, these results support the efficacy of LDX from 1.5 hours to 13 hours postdose in boys and girls with medium to large effect sizes across the day with some variability in TEAE incidence by sex.
Trial registration number: ClinicalTrials.gov Identifier: NCT00500149.
Figures

References
-
- Pliszka SR, Crismon ML, Hughes CW, Conners CK, Emslie GJ, Jensen PS, McCracken JT, Swanson JM, Lopez M. The Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention-Deficit/Hyperactivity Disorder. The Texas Children's Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:642–657. doi: 10.1097/01.chi.0000215326.51175.eb. - DOI - PubMed
-
- Stein MA, Baren M. Welcome progress in the diagnosis and treatment of ADHD in adolescence. Contemp Pediatr. 2003;20:83–110.
-
- Stein MA. Innovations in attention-deficit/hyperactivity disorder pharmacotherapy: long-acting stimulant and nonstimulant treatments. Am J Manag Care. 2004;10(suppl):S89–S98. - PubMed

