Voltage-regulated water flux through aquaporin channels in silico
- PMID: 21156125
- PMCID: PMC3000486
- DOI: 10.1016/j.bpj.2010.11.003
Voltage-regulated water flux through aquaporin channels in silico
Abstract
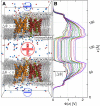
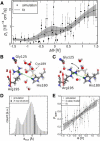
Aquaporins (AQPs) facilitate the passive flux of water across biological membranes in response to an osmotic pressure. A number of AQPs, for instance in plants and yeast, have been proposed to be regulated by phosphorylation, cation concentration, pH change, or membrane-mediated mechanical stress. Here we report an extensive set of molecular dynamics simulations of AQP1 and AQP4 subject to large membrane potentials in the range of ±1.5 V, suggesting that AQPs may in addition be regulated by an electrostatic potential. As the regulatory mechanism we identified the relative population of two different states of the conserved arginine in the aromatic/arginine constriction region. A positive membrane potential was found to stabilize the arginine in an up-state, which allows rapid water flux, whereas a negative potential favors a down-state, which reduces the single-channel water permeability.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Preston G.M., Carroll T.P., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. - PubMed
-
- King L.S., Kozono D., Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. - PubMed
-
- Törnroth-Horsefield S., Hedfalk K., Neutze R. Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 2010;584:2580–2588. - PubMed
-
- Nedvetsky P.I., Tamma G., Klussmann E. Regulation of aquaporin-2 trafficking. Handb. Exp. Pharmacol. 2009;190:133–157. - PubMed
-
- Wang Y., Schulten K., Tajkhorshid E. What makes an aquaporin a glycerol channel? A comparative study of AqpZ and GlpF. Structure. 2005;13:1107–1118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

