Ethanol-stimulated differentiated functions of human or mouse hepatic stellate cells are mediated by connective tissue growth factor
- PMID: 21156189
- PMCID: PMC3136646
- DOI: 10.1016/j.jhep.2010.11.025
Ethanol-stimulated differentiated functions of human or mouse hepatic stellate cells are mediated by connective tissue growth factor
Abstract
Background & aims: Connective tissue growth factor (CTGF) expression is intimately associated with hepatic fibrotic pathophysiology. In this study, CTGF production and action was investigated in ethanol-treated mouse primary hepatic stellate cells (HSC) or human LX-2 cells.
Methods: CTGF, transforming growth factor-beta1 (TGF-β1), alpha-smooth muscle actin (α-SMA) or collagen α1(I) mRNA were quantified by real-time PCR after treatment of HSC with ethanol or acetaldehyde. CTGF protein production was assessed by immunoprecipitation or ELISA. Ethanol-stimulated CTGF transcription was investigated using CTGF promoter reporter constructs. The TGF-β1- or CTGF-dependency of ethanol-induced CTGF, α-SMA, or collagen α1(I) was determined using small interfering RNA (siRNA) to TGF-β1 or CTGF.
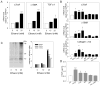
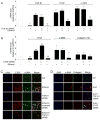
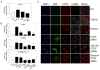
Results: In human steatohepatitis, CTGF was produced by presumptive activated HSC. In cultured human or mouse HSC, production of CTGF, α-SMA and/or collagen was increased by ethanol treatment, an effect mimicked by acetaldehyde and blocked by 4-methylpyrazole (4-MP) or N-acetylcysteine (NAC). CTGF promoter activity was stimulated in a sustained fashion by ethanol or TGF-β1. Mutation of the Smad site or basal control element (BCE-1) in the CTGF promoter caused a 5-fold reduction in ethanol-stimulated CTGF promoter activity. Administration of TGF-β1 siRNA or CTGF siRNA significantly decreased ethanol- or acetaldehyde-stimulated mRNA or protein levels of CTGF, α-SMA or collagen I in LX-2 cells. In mouse HSC, TGF-β1- or ethanol-stimulated CTGF, α-SMA or collagen I were significantly attenuated by CTGF siRNA.
Conclusions: Ethanol-induced α-SMA or collagen α1(I) in HSC are mediated via TGF-β-dependent CTGF production, highlighting potential therapeutic benefits of targeting CTGF in alcoholic liver disease.
Copyright © 2010 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Crawford JM. Liver Cirrhosis. In: MacSween RNM, Burt AD, Portmann BC, Ishak KG, Scheuer PJ, Anthony PP, editors. Pathology of the Liver. Harcourt Publishers Limited; London: 2002. pp. 575–619.
-
- Friedman SL. Hepatic fibrosis -- overview. Toxicology. 2008;254:120–129. - PubMed
-
- Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–807. - PubMed
-
- Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26:1–9. - PubMed
-
- Williams EJ, Gaca MD, Brigstock DR, Arthur MJ, Benyon RC. Increased expression of connective tissue growth factor in fibrotic human liver and in activated hepatic stellate cells. J Hepatol. 2000;32:754–761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

