RNA interference for improving the outcome of islet transplantation
- PMID: 21156190
- PMCID: PMC3065652
- DOI: 10.1016/j.addr.2010.11.003
RNA interference for improving the outcome of islet transplantation
Abstract
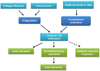
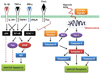
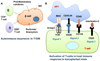
Islet transplantation has the potential to cure type 1 diabetes. Despite recent therapeutic success, it is still not common because a large number of transplanted islets get damaged by multiple challenges including instant blood mediated inflammatory reaction, hypoxia/reperfusion injury, inflammatory cytokines, and immune rejection. RNA interference (RNAi) is a novel strategy to selectively degrade target mRNA. The use of RNAi technologies to downregulate the expression of harmful genes has the potential to improve the outcome of islet transplantation. The aim of this review is to gain a thorough understanding of biological obstacles to islet transplantation and discuss how to overcome these barriers using different RNAi technologies. This eventually will help improve islet survival and function post transplantation. Chemically synthesized small interferring RNA (siRNA), vector based short hairpin RNA (shRNA), and their critical design elements (such as sequences, promoters, and backbone) are discussed. The application of combinatorial RNAi in islet transplantation is also discussed. Last but not the least, several delivery strategies for enhanced gene silencing are discussed, including chemical modification of siRNA, complex formation, bioconjugation, and viral vectors.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures






References
-
- Williams PW. Notes on diabetes treated with extract and by grafts of sheep’s pancreas. Br Med J. 1894;2:1303–1304.
-
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61:827–837. - PubMed
-
- Vardanyan M, Parkin E, Gruessner C, Rodriguez Rilo HL. Pancreas vs. islet transplantation: a call on the future. Curr Opin Organ Transplant. 2010;15:124–130. - PubMed
-
- Troppmann C. Complications after pancreas transplantation. Curr Opin Organ Transplant. 2010;15:112–118. - PubMed
-
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

