Impaired myocardial perfusion reserve and fibrosis in Friedreich ataxia: a mitochondrial cardiomyopathy with metabolic syndrome
- PMID: 21156720
- PMCID: PMC3106287
- DOI: 10.1093/eurheartj/ehq443
Impaired myocardial perfusion reserve and fibrosis in Friedreich ataxia: a mitochondrial cardiomyopathy with metabolic syndrome
Abstract
Aims: Cardiomyopathy produces significant mortality in patients with Friedreich ataxia (FA), a genetic disorder that produces intra-mitochondrial iron accumulation. We sought to test the hypothesis that abnormal myocardial perfusion reserve and fibrosis represent early manifestations of cardiomyopathy.
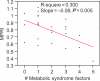
Methods and results: Twenty-six patients with genetically proven FA ages 36 ± 12 years without cardiomyopathy and eight controls underwent cardiac magnetic resonance with adenosine. Precontrast imaging for myocardial iron estimation was performed. Myocardial perfusion reserve index (MPRI) was quantified using the normalized upslope of myocardial enhancement during vasodilator stress vs. rest. Left ventricular (LV) mass and volumes were computed from short-axis cine images. Serologies included lipids, and platelets were isolated for iron quantification using inductively coupled plasma mass spectrometry. Left ventricular ejection fraction and mass averaged 64.1 ± 8.3% and 62.7 ± 16.7 g/m², respectively, indicating preserved systolic function and absence of significant hypertrophy. Myocardial perfusion reserve index quantification revealed significantly lower endocardial-to-epicardial perfusion reserve in patients vs. controls (0.80 ± 0.18 vs. 1.22 ± 0.36, P = 0.01). Lower MPRI was predicted by increased number of metabolic syndrome (met-S) features (P < 0.01). Worse concentric remodelling occurred with increased GAA repeat length (r = 0.64, P < 0.001). Peripheral platelet iron measurement showed no distinction between patients and controls (5.4 ± 8.5 × 10⁻⁷ vs. 5.5 ± 2.9 × 10⁻⁷ ng/platelet, P = 0.88), nor did myocardial T2* measures.
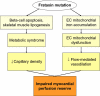
Conclusions: Patients with FA have abnormal myocardial perfusion reserve that parallels met-S severity. Impaired perfusion reserve and fibrosis occur in the absence of significant hypertrophy and prior to clinical heart failure, providing potential therapeutic targets for stage B cardiomyopathy in FA and related myocardial diseases.
Figures


Similar articles
-
Serum versus Imaging Biomarkers in Friedreich Ataxia to Indicate Left Ventricular Remodeling and Outcomes.Tex Heart Inst J. 2016 Aug 1;43(4):305-10. doi: 10.14503/THIJ-14-4198. eCollection 2016 Aug. Tex Heart Inst J. 2016. PMID: 27547137 Free PMC article.
-
Relation of Myocardial Perfusion Reserve and Left Ventricular Ejection Fraction in Ischemic and Nonischemic Cardiomyopathy.Am J Cardiol. 2022 Jul 1;174:143-150. doi: 10.1016/j.amjcard.2022.02.022. Epub 2022 Apr 26. Am J Cardiol. 2022. PMID: 35487776 Free PMC article.
-
Extracellular volume fraction is more closely associated with altered regional left ventricular velocities than left ventricular ejection fraction in nonischemic cardiomyopathy.Circ Cardiovasc Imaging. 2014 Dec 31;8(1):e001998. doi: 10.1161/CIRCIMAGING.114.001998. Print 2015 Jan. Circ Cardiovasc Imaging. 2014. PMID: 25552491 Free PMC article.
-
Myocardial Perfusion Imaging with Cardiovascular Magnetic Resonance in Nonischemic Cardiomyopathies: An In-Depth Review of Techniques and Clinical Applications.Medicina (Kaunas). 2025 May 10;61(5):875. doi: 10.3390/medicina61050875. Medicina (Kaunas). 2025. PMID: 40428833 Free PMC article. Review.
-
Cardiovascular Research in Friedreich Ataxia: Unmet Needs and Opportunities.JACC Basic Transl Sci. 2022 Jul 13;7(12):1267-1283. doi: 10.1016/j.jacbts.2022.04.005. eCollection 2022 Dec. JACC Basic Transl Sci. 2022. PMID: 36644283 Free PMC article. Review.
Cited by
-
Investigation of cardiomyopathy using cardiac magnetic resonance imaging part 2: Rare phenotypes.World J Cardiol. 2012 May 26;4(5):173-82. doi: 10.4330/wjc.v4.i5.173. World J Cardiol. 2012. PMID: 22655165 Free PMC article.
-
Left ventricular structural and functional changes in Friedreich ataxia - Relationship with body size, sex, age and genetic severity.PLoS One. 2019 Nov 13;14(11):e0225147. doi: 10.1371/journal.pone.0225147. eCollection 2019. PLoS One. 2019. PMID: 31721791 Free PMC article.
-
Microvascular pathology in Friedreich cardiomyopathy.Histol Histopathol. 2020 Jan;35(1):39-46. doi: 10.14670/HH-18-132. Epub 2019 Jun 5. Histol Histopathol. 2020. PMID: 31166002
-
Heart disease in Friedreich's ataxia.World J Cardiol. 2019 Jan 26;11(1):1-12. doi: 10.4330/wjc.v11.i1.1. World J Cardiol. 2019. PMID: 30705738 Free PMC article. Review.
-
Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's ataxia.Nat Med. 2014 May;20(5):542-7. doi: 10.1038/nm.3510. Epub 2014 Apr 6. Nat Med. 2014. PMID: 24705334
References
-
- Ropper AH, Samuels MA. Adams and Victor's Principles of Neurology. 9th Edition. USA: The McGraw-Hill Companies, Inc.; 2009.
-
- Child JS, Perloff JK, Bach PM, Wolfe AD, Perlman S, Kark RA. Cardiac involvement in Friedreich's ataxia: a clinical study of 75 patients. J Am Coll Cardiol. 1986;7:1370–1378. doi:10.1016/S0735-1097(86)80159-0. - DOI - PubMed
-
- Meyer C, Schmid G, Gorlitz S, Ernst M, Wilkens C, Wilhelms I, Kraus PH, Bauer P, Tomiuk J, Przuntek H, Mugge A, Schols L. Cardiomyopathy in Friedreich's ataxia-assessment by cardiac MRI. Mov Disord. 2007;22:1615–1622. doi:10.1002/mds.21590. - DOI - PubMed
-
- Delatycki MB, Williamson R, Forrest SM. Friedreich ataxia: an overview. J Med Genet. 2000;37:1–8. doi:10.1136/jmg.37.1.1. - DOI - PMC - PubMed
-
- Pandolfo M. Friedreich ataxia. Arch Neurol. 2008;65:1296–1303. doi:10.1001/archneur.65.10.1296. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

