Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK
- PMID: 21156789
- PMCID: PMC3037668
- DOI: 10.1074/jbc.M110.183699
Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK
Retraction in
-
Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors. EVIDENCE FOR THE ROLE OF REACTIVE OXYGEN SPECIES AND JNK.J Biol Chem. 2016 Aug 5;291(32):16924. doi: 10.1074/jbc.A110.183699. J Biol Chem. 2016. PMID: 27496965 Free PMC article. No abstract available.
Abstract
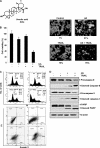
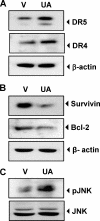
Discovery of the molecular targets of traditional medicine and its chemical footprints can validate the use of such medicine. In the present report, we investigated the effect of ursolic acid (UA), a pentacyclic triterpenoid found in rosemary and holy basil, on apoptosis induced by TRAIL. We found that UA potentiated TRAIL-induced apoptosis in cancer cells. In addition, UA also sensitized TRAIL-resistant cancer cells to the cytokine. When we investigated the mechanism, we found that UA down-regulated cell survival proteins and induced the cell surface expression of both TRAIL receptors, death receptors 4 and 5 (DR4 and -5). Induction of receptors by UA occurred independently of cell type. Gene silencing of either receptor by small interfering RNA reduced the apoptosis induced by UA and the effect of TRAIL. In addition, UA also decreased the expression of decoy receptor 2 (DcR2) but not DcR1. Induction of DRs was independent of p53 because UA induced DR4 and DR5 in HCT116 p53(-/-) cells. Induction of DRs, however, was dependent on JNK because UA induced JNK, and its pharmacologic inhibition abolished the induction of the receptors. The down-regulation of survival proteins and up-regulation of the DRs required reactive oxygen species (ROS) because UA induced ROS, and its quenching abolished the effect of the terpene. Also, potentiation of TRAIL-induced apoptosis by UA was significantly reduced by both ROS quenchers and JNK inhibitor. In addition, UA was also found to induce the expression of DRs, down-regulate cell survival proteins, and activate JNK in orthotopically implanted human colorectal cancer in a nude mouse model. Overall, our results showed that UA potentiates TRAIL-induced apoptosis through activation of ROS and JNK-mediated up-regulation of DRs and down-regulation of DcR2 and cell survival proteins.
Figures






References
-
- Butler M. S., Newman D. J. (2008) Prog. Drug Res. 65, 3–44 - PubMed
-
- Liu J. (1995) J. Ethnopharmacol. 49, 57–68 - PubMed
-
- Aggarwal B. B. (2003) Nat. Rev. Immunol. 3, 745–756 - PubMed
-
- Es-saady D., Simon A., Ollier M., Maurizis J. C., Chulia A. J., Delage C. (1996) Cancer Lett. 106, 193–197 - PubMed
-
- Hsu Y. L., Kuo P. L., Lin C. C. (2004) Life Sci. 75, 2303–2316 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

