The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation
- PMID: 21156980
- PMCID: PMC4327979
- DOI: 10.1213/ANE.0b013e31820568af
The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation
Abstract
Background: The possibility that μ opioid agonists can influence cancer recurrence is a subject of recent interest. Epidemiologic studies suggested that there were differences in cancer recurrence in breast and prostate cancer contingent on anesthetic regimens. In this study, we identify a possible mechanism for these epidemiologic findings on the basis of μ opioid receptor (MOR) regulation of Lewis lung carcinoma (LLC) tumorigenicity in cell and animal models.
Methods: We used human lung tissue and human non-small cell lung cancer (NSCLC) cell lines and evaluated MOR expression using immunoblot and immunohistochemical analysis. LLC cells were treated with the peripheral opioid antagonist methylnaltrexone (MNTX) or MOR shRNA and evaluated for proliferation, invasion, and soft agar colony formation in vitro and primary tumor growth and lung metastasis in C57BL/6 and MOR knockout mice using VisEn fluorescence mediated tomography imaging and immunohistochemical analysis.
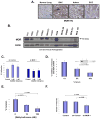
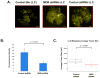
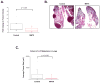
Results: We provide several lines of evidence that the MOR may be a potential target for lung cancer, a disease with high mortality and few treatment options. We first observed that there is ∼5- to 10-fold increase in MOR expression in lung samples from patients with NSCLC and in several human NSCLC cell lines. The MOR agonists morphine and [D-Ala(2), N-MePhe(4), Gly-ol]-enkephalin (DAMGO) increased in vitro LLC cell growth. Treatment with MNTX or silencing MOR expression inhibited LLC invasion and anchorage-independent growth by 50%-80%. Injection of MOR silenced LLC lead to a ∼65% reduction in mouse lung metastasis. In addition, MOR knockout mice do not develop significant tumors when injected with LLC in comparison with wild-type controls. Finally, continuous infusion of the peripheral opioid antagonist MNTX attenuates primary LLC tumor growth and reduces lung metastasis.
Conclusions: Taken together, our data suggest a possible direct effect of opiates on lung cancer progression, and provide a plausible explanation for the epidemiologic findings. Our observations further suggest a possible therapeutic role for opioid antagonists.
Conflict of interest statement
Dr. Moss serves as a paid consultant to Progenics Pharmaceuticals, Inc., has a financial interest in methylnaltrexone as a patent holder through the University of Chicago, and receives stock options from Progenics.
Figures

References
-
- Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110:1636–1643. - PubMed
-
- Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–187. - PubMed
-
- Moss J, Israel RJ. Effects of anesthetics on cancer recurrence. J Clin Oncol. 2009;27:e89. author reply e90. - PubMed
-
- Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: Role of receptor transactivation. Microvasc Res. 2006;72:3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

