Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rγc-deficient mice
- PMID: 21157036
- PMCID: PMC3007135
- DOI: 10.1172/JCI41495
Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rγc-deficient mice
Abstract
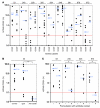
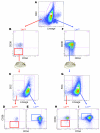
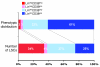
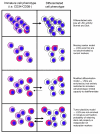
Human leukemic stem cells, like other cancer stem cells, are hypothesized to be rare, capable of incomplete differentiation, and restricted to a phenotype associated with early hematopoietic progenitors or stem cells. However, recent work in other types of tumors has challenged the cancer stem cell model. Using a robust model of xenotransplantation based on NOD/SCID/IL2Rγc-deficient mice, we confirmed that human leukemic stem cells, functionally defined by us as SCID leukemia-initiating cells (SL-ICs), are rare in acute myelogenous leukemia (AML). In contrast to previous results, SL-ICs were found among cells expressing lineage markers (i.e., among Lin+ cells), CD38, or CD45RA, all markers associated with normal committed progenitors. Remarkably, each engrafting fraction consistently recapitulated the original phenotypic diversity of the primary AML specimen and contained self-renewing leukemic stem cells, as demonstrated by secondary transplants. While SL-ICs were enriched in the Lin-CD38- fraction compared with the other fractions analyzed, SL-ICs in this fraction represented only one-third of all SL-ICs present in the unfractionated specimen. These results indicate that human AML stem cells are rare and enriched but not restricted to the phenotype associated with normal primitive hematopoietic cells. These results suggest a plasticity of the cancer stem cell phenotype that we believe has not been previously described.
Figures




References
-
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

