T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis
- PMID: 21157437
- PMCID: PMC3048186
- DOI: 10.1038/mt.2010.272
T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis
Abstract
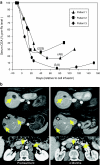
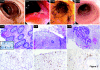
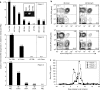
Autologous T lymphocytes genetically engineered to express a murine T cell receptor (TCR) against human carcinoembryonic antigen (CEA) were administered to three patients with metastatic colorectal cancer refractory to standard treatments. All patients experienced profound decreases in serum CEA levels (74-99%), and one patient had an objective regression of cancer metastatic to the lung and liver. However, a severe transient inflammatory colitis that represented a dose limiting toxicity was induced in all three patients. This report represents the first example of objective regression of metastatic colorectal cancer mediated by adoptive T cell transfer and illustrates the successful use of a TCR, raised in human leukocyte antigen (HLA) transgenic mice, against a human tumor associated antigen. It also emphasizes the destructive power of small numbers of highly avid T cells and the limitations of using CEA as a target for cancer immunotherapy.
Figures
References
-
- Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. - PubMed
-
- Foon KA, John WJ, Chakraborty M, Das R, Teitelbaum A, Garrison J, et al. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J Clin Oncol. 1999;17:2889–2885. - PubMed
-
- Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

