Flow measurement in MRI using arterial spin labeling with cumulative readout pulses--theory and validation
- PMID: 21158292
- PMCID: PMC2973993
- DOI: 10.1118/1.3501881
Flow measurement in MRI using arterial spin labeling with cumulative readout pulses--theory and validation
Abstract
Purpose: This article systematically examines arterial spin labeling (ASL) as a flow quantification technique through theoretical simulation, in vitro, and in vivo experiment. The authors present a novel imaging pulse sequence design consisting of a single ASL magnetization preparation followed by Look-Locker-like image readouts. Bloch-equation-based modeling has been developed and validated using a hemodialyzer as a tissue-mimicking flow phantom.
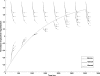
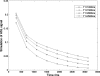
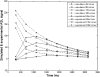
Methods: After the single in-plane slice-selective double inversion magnetization preparation, multiple TFL readouts are acquired with linear k-space ordering, causing a signal variation that depends on through-slice flow velocity. Computer simulations were performed to assess the behavior of the flow-dependent ASL signal as a function of varying imaging parameters. The signal was optimized by choosing imaging parameters that maximize the simulated flow-sensitive signal. Furthermore, a hemodialyzer which mimics blood flow in human tissues was tested with a wide range of flow rates. An exponential curve fitting of the flow-sensitive dynamics to the model derived from Bloch equations provides a method to estimate through-slice velocity for varying flow rates on the hemodialyzer and in vivo human brain.
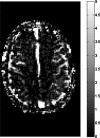
Results: The flow dependency of the ASL signal and the sensitivity of the ASL signal to imaging parameters were demonstrated. Experimental results from a hemodialyzer when fitted with a Bloch-equation-based model provide flow measurements that are consistent with ground truth velocities. Human brain velocity mapping was obtained as well.
Conclusions: The results provide evidence that the proposed pulse sequence design is an effective technique to measure total fluid flow through image voxels. The unique combination of the two main features, multiple-image readout after a single ASL preparation and linear acquisition ordering in the phase encoding direction in TFL imaging, make this technique an appealing flow imaging method to quantify through-plane flow in a time-efficient manner.
Figures






References
-
- Song C. W., Rhee J. G., and Levitt S. H., “Blood flow in normal tissues and tumors during hyperthermia,” J. Natl. Cancer Inst. JNCIEQ 64(1), 119–124 (1980). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

