Optimization of a dual-energy contrast-enhanced technique for a photon-counting digital breast tomosynthesis system: I. A theoretical model
- PMID: 21158302
- PMCID: PMC3188980
- DOI: 10.1118/1.3490556
Optimization of a dual-energy contrast-enhanced technique for a photon-counting digital breast tomosynthesis system: I. A theoretical model
Abstract
Purpose: Dual-energy (DE) iodine contrast-enhanced x-ray imaging of the breast has been shown to identify cancers that would otherwise be mammographically occult. In this article, theoretical modeling was performed to obtain optimally enhanced iodine images for a photon-counting digital breast tomosynthesis (DBT) system using a DE acquisition technique.
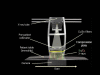
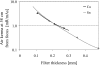
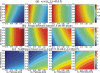
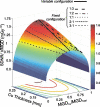
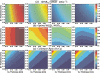
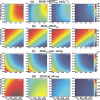
Methods: In the system examined, the breast is scanned with a multislit prepatient collimator aligned with a multidetector camera. Each detector collects a projection image at a unique angle during the scan. Low-energy (LE) and high-energy (HE) projection images are acquired simultaneously in a single scan by covering alternate collimator slits with Sn and Cu filters, respectively. Sn filters ranging from 0.08 to 0.22 mm thickness and Cu filters from 0.11 to 0.27 mm thickness were investigated. A tube voltage of 49 kV was selected. Tomographic images, hereafter referred to as DBT images, were reconstructed using a shift-and-add algorithm. Iodine-enhanced DBT images were acquired by performing a weighted logarithmic subtraction of the HE and LE DBT images, The DE technique was evaluated for 20-80 mm thick breasts. Weighting factors, w(t) that optimally cancel breast tissue were computed. Signal-difference-to-noise ratios (SDNRs) between iodine-enhanced and nonenhanced breast tissue normalized to the square root of the mean glandular dose (MGD) were computed as a function of the fraction of the MGD allocated to the HE images. Peak SDNR/ mean square root of MGD and optimal dose allocations were identified. SDNR/ mean square root of MGD and dose allocations were computed for several practical feasible system configurations (i.e., determined by the number of collimator slits covered by Sn and Cu). A practicalsystem configuration an d Sn-Cu filterpair that accounts for the trade-off between SDNR, tube-output, and MGD were selected.
Results: w(t) depends on the Sn-Cu filter combination used, as well as on the breast thickness; to optimally cancel 0% with 50% glandular breast tissue, w(t) values were found to range from 0.46 to 0.72 for all breast thicknesses and Sn-Cu filter pairs studied. The optimal w(t) values needed to cancel all possible breast tissue glandularites vary by less than 1% for 20 mm thick breasts and 18% for 80 mm breasts. The system configuration where one collimator slit covered by Sn is alternated with two collimator slits covered by Cu delivers SDNR/ mean square root of MGD nearest to the peak value. A reasonable compromise is a 0.16 mm Sn-0.23 mm Cu filter pair, resulting in SDNR values between 1.64 and 0.61 and MGD between 0.70 and 0.53 mGy for 20-80 mm thick breasts at the maximum tube current.
Conclusions: A DE acquisition technique for a photon-counting DBT imaging system has been developed and optimized.
Figures



References
-
- Hylton N. M., “Vascularity assessment of breast lesions with gadolinium-enhanced MR imaging,” Magn. Reson Imaging Clin. N. Am. ZZZZZZ 9, 321–331 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

