The C-terminal domain of the neutral amino acid transporter SNAT2 regulates transport activity through voltage-dependent processes
- PMID: 21158741
- PMCID: PMC3102179
- DOI: 10.1042/BJ20100507
The C-terminal domain of the neutral amino acid transporter SNAT2 regulates transport activity through voltage-dependent processes
Abstract
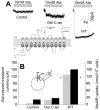
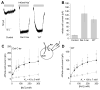
SNAT (sodium-coupled neutral amino acid transporter) 2 belongs to the SLC38 (solute carrier 38) family of solute transporters. Transport of one amino acid molecule into the cell is driven by the co-transport of one Na(+) ion. The functional significance of the C-terminus of SNAT2, which is predicted to be located in the extracellular space, is currently unknown. In the present paper, we removed 13 amino acid residues from the SNAT2 C-terminus and studied the effect of this deletion on transporter function. The truncation abolished amino acid transport currents at negative membrane potentials (<0 mV), as well as substrate uptake. However, transport currents were observed at positive membrane potentials demonstrating that transport was accelerated while the driving force decreased. Membrane expression levels were normal in the truncated transporter. SNAT2(Del C-ter) (13 residues deleted from the C-terminus) showed 3-fold higher apparent affinity for alanine, and 2-fold higher Na(+) affinity compared with wild-type SNAT2, suggesting that the C-terminus is not required for high-affinity substrate and Na(+) interaction with SNAT2. The pH sensitivity of amino acid transport was retained partially after the truncation. In contrast with the truncation after TM (transmembrane domain) 11, the deletion of TM11 resulted in an inactive transporter, most probably due to a defect in cell surface expression. Taken together, the results demonstrate that the C-terminal domain of SNAT2 is an important voltage regulator that is required for a normal amino acid translocation process at physiological membrane potentials. However, the C-terminus appears not to be involved in the regulation of membrane expression.
© The Authors Journal compilation © 2011 Biochemical Society
Figures

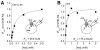
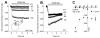


References
-
- Sugawara M, Nakanishi T, Fei YJ, Huang W, Ganapathy ME, Leibach FH, Ganapathy V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J Biol Chem. 2000;275:16473–16477. - PubMed
-
- Yao D, Mackenzie B, Ming H, Varoqui H, Zhu H, Hediger MA, Erickson JD. A novel system A isoform mediating Na+/neutral amino acid cotransport. J Biol Chem. 2000;275:22790–22797. - PubMed
-
- Hatanaka T, Huang W, Wang H, Sugawara M, Prasad PD, Leibach FH, Ganapathy V. Primary structure, functional characteristics and tissue expression pattern of human ATA2, a subtype of amino acid transport system A. Biochim Biophys Acta. 2000;1467:1–6. - PubMed
-
- Varoqui H, Zhu H, Yao D, Ming H, Erickson JD. Cloning and functional identification of a neuronal glutamine transporter. J Biol Chem. 2000;275:4049–4054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

