Structural basis for three-step sequential catalysis by the cholesterol side chain cleavage enzyme CYP11A1
- PMID: 21159775
- PMCID: PMC3037674
- DOI: 10.1074/jbc.M110.188433
Structural basis for three-step sequential catalysis by the cholesterol side chain cleavage enzyme CYP11A1
Abstract
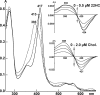
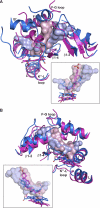
Mitochondrial cytochrome P450 11A1 (CYP11A1 or P450 11A1) is the only known enzyme that cleaves the side chain of cholesterol, yielding pregnenolone, the precursor of all steroid hormones. Pregnenolone is formed via three sequential monooxygenation reactions that involve the progressive production of 22R-hydroxycholesterol (22HC) and 20α,22R-dihydroxycholesterol, followed by the cleavage of the C20-C22 bond. Herein, we present the 2.5-Å crystal structure of CYP11A1 in complex with the first reaction intermediate, 22HC. The active site cavity in CYP11A1 represents a long curved tube that extends from the protein surface to the heme group, the site of catalysis. 22HC occupies two-thirds of the cavity with the 22R-hydroxyl group nearest the heme, 2.56 Å from the iron. The space at the entrance to the active site is not taken up by 22HC but filled with ordered water molecules. The network formed by these water molecules allows the "soft" recognition of the 22HC 3β-hydroxyl. Such a mode of 22HC binding suggests shuttling of the sterol intermediates between the active site entrance and the heme group during the three-step reaction. Translational freedom of 22HC and torsional motion of its aliphatic tail are supported by solution studies. The CYP11A1-22HC co-complex also provides insight into the structural basis of the strict substrate specificity and high catalytic efficiency of the enzyme and highlights conserved structural motifs involved in redox partner interactions by mitochondrial P450s.
Figures

References
-
- Simpson E. R., Boyd G. S. (1967) Eur. J. Biochem. 2, 275–285 - PubMed
-
- Burstein S., Middleditch B. S., Gut M. (1975) J. Biol. Chem. 250, 9028–9037 - PubMed
-
- Orme-Johnson N. R., Light D. R., White-Stevens R. W., Orme-Johnson W. H. (1979) J. Biol. Chem. 254, 2103–2111 - PubMed
-
- Lambeth J. D., Kitchen S. E., Farooqui A. A., Tuckey R., Kamin H. (1982) J. Biol. Chem. 257, 1876–1884 - PubMed
-
- Pikuleva I. A. (2006) Drug Metab. Dispos. 34, 513–520 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

