The unfolded protein response in familial amyotrophic lateral sclerosis
- PMID: 21159797
- PMCID: PMC3033190
- DOI: 10.1093/hmg/ddq546
The unfolded protein response in familial amyotrophic lateral sclerosis
Abstract
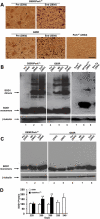
Mutant superoxide dismutase type 1 (MTSOD1) is thought to cause ∼20% of cases of familial amyotrophic lateral sclerosis (FALS) because it misfolds and aggregates. Previous studies have shown that MTSOD1 accumulates inside the endoplasmic reticulum (ER) and activates the unfolded protein response (UPR), suggesting that ER stress is involved in the pathogenesis of FALS. We used a genetic approach to investigate the role of the UPR in FALS. We crossed G85RSOD1 transgenic mice with pancreatic ER kinase haploinsufficient (PERK(+/-)) mice to obtain G85R/PERK(+/-) mice. PERK(+/-) mice carry a loss of function mutation of PERK, which is the most rapidly activated UPR pathway, but have no abnormal phenotype. Compared with G85R transgenic mice, G85R/PERK(+/-) mice had a dramatically accelerated disease onset as well as shortened disease duration and lifespan. There was also acceleration of the pathology and earlier MTSOD1 aggregation. A diminished PERK response accelerated disease and pathology in G85R transgenic mice presumably because the mice had a reduced capacity to turn down synthesis of misfolded SOD1, leading to an early overloading of the UPR. The results indicate that the UPR has a significant influence on FALS, and suggest that enhancing the UPR may be effective in treating ALS.
Figures



References
-
- Rothstein J.D. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann. Neurol. 2009;65:S3–S9. doi:10.1002/ana.21543. - DOI - PubMed
-
- Boillee S., Vande Velde C., Cleveland D.W. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi:10.1016/j.neuron.2006.09.018. - DOI - PubMed
-
- Wang L., Sharma K., Grisotti G., Roos R.P. The effect of mutant SOD1 dismutase activity on non-cell autonomous degeneration in familial amyotrophic lateral sclerosis. Neurobiol. Dis. 2009;35:234–240. doi:10.1016/j.nbd.2009.05.002. - DOI - PMC - PubMed
-
- Wang L., Gutmann D.H., Roos R.P. Astrocyte loss of mutant SOD1 delays ALS disease onset and progression in G85R transgenic mice. Hum. Mol. Genet. 2010 in press. - PubMed
-
- Zhang K., Kaufman R.J. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–S109. doi:10.1212/01.wnl.0000192306.98198.ec. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

