TOPORS, implicated in retinal degeneration, is a cilia-centrosomal protein
- PMID: 21159800
- PMCID: PMC3033188
- DOI: 10.1093/hmg/ddq543
TOPORS, implicated in retinal degeneration, is a cilia-centrosomal protein
Abstract
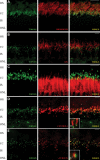
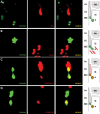
We recently reported that mutations in the widely expressed nuclear protein TOPORS (topoisomerase I-binding arginine/serine rich) are associated with autosomal dominant retinal degeneration. However, the precise localization and a functional role of TOPORS in the retina remain unknown. Here, we demonstrate that TOPORS is a novel component of the photoreceptor sensory cilium, which is a modified primary cilium involved with polarized trafficking of proteins. In photoreceptors, TOPORS localizes primarily to the basal bodies of connecting cilium and in the centrosomes of cultured cells. Morpholino-mediated silencing of topors in zebrafish embryos demonstrates in another species a comparable retinal problem as seen in humans, resulting in defective retinal development and failure to form outer segments. These defects can be rescued by mRNA encoding human TOPORS. Taken together, our data suggest that TOPORS may play a key role in regulating primary cilia-dependent photoreceptor development and function. Additionally, it is well known that mutations in other ciliary proteins cause retinal degeneration, which may explain why mutations in TOPORS result in the same phenotype.
Figures




References
-
- Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi:10.1016/S0140-6736(06)69740-7. - DOI - PubMed
-
- Wright A.F., Chakarova C.F., Abd El-Aziz M.M., Bhattacharya S.S. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 2010;11:273–284. doi:10.1038/nrg2717. - DOI - PubMed
-
- Rivolta C., Sharon D., DeAngelis M.M., Dryja T.P. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum. Mol. Genet. 2002;11:1219–1227. doi:10.1093/hmg/11.10.1219. - DOI - PubMed
-
- Chakarova C.F., Papaioannou M.G., Khanna H., Lopez I., Waseem N., Shah A., Theis T., Friedman J., Maubaret C., Bujakowska K., et al. Mutations in TOPORS cause autosomal dominant retinitis pigmentosa with perivascular RPE atrophy. Am. J. Hum. Genet. 2007;81:1098–1103. doi:10.1086/521953. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

