NADPH oxidase-derived reactive oxygen species mediate decidualization of human endometrial stromal cells in response to cyclic AMP signaling
- PMID: 21159852
- PMCID: PMC3037160
- DOI: 10.1210/en.2010-0899
NADPH oxidase-derived reactive oxygen species mediate decidualization of human endometrial stromal cells in response to cyclic AMP signaling
Abstract
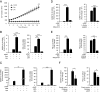
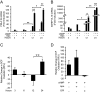
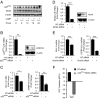
Differentiation of human endometrial stromal cells into specialized decidual cells is critical for embryo implantation and survival of the conceptus. Initiation of this differentiation process is strictly dependent on elevated cAMP levels, but the signal intermediates that control the expression of decidual marker genes, such as prolactin (PRL) and IGFBP1, remain poorly characterized. Here we show that cAMP-dependent decidualization can be attenuated or enhanced upon treatment of primary cultures with a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor (diphenylen iodonium) or activator (apocynin), respectively. Time-course analysis demonstrated that cAMP enhances endogenous reactive oxygen species production, apparent after 12 h of stimulation, which coincides with a dramatic increase in decidual PRL and IGFBP1 expression. Knockdown of the Rho GTPase RAC1, which disables activation of the NADPH oxidase homologs NADPH oxidase (NOX)-1, NOX-2, and NOX-3, had no effect on PRL or IGFBP1 expression. In contrast, silencing of NOX-4, or its cofactor p22(PHOX), inhibited the expression of both decidual markers. Finally, we show that the NOX-4/p22(PHOX) complex regulates the DNA-binding activity of CCAAT/enhancer binding protein-β, a key regulator of human endometrial stromal cell differentiation. Thus, NOX-4 activation and reactive oxygen species signaling play an integral role in initiating the endometrial decidual response in preparation of pregnancy.
Figures


References
-
- Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Macklon NS, Brosens JJ. 2010. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One 5:e10287. - PMC - PubMed
-
- Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Brosens JJ, Macklon NS. 2010. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One 5:e10258. - PMC - PubMed
-
- Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM. 2010. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum Reprod 25:862–873 - PubMed
-
- Kajihara T, Jones M, Fusi L, Takano M, Feroze-Zaidi F, Pirianov G, Mehmet H, Ishihara O, Higham JM, Lam EW, Brosens JJ. 2006. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol Endocrinol 20:2444–2455 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

