Reimagining Alzheimer's disease--an age-based hypothesis
- PMID: 21159946
- PMCID: PMC3004746
- DOI: 10.1523/JNEUROSCI.4521-10.2010
Reimagining Alzheimer's disease--an age-based hypothesis
Abstract
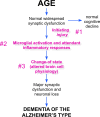
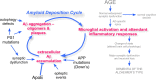
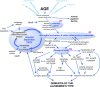
The historical roots of Alzheimer's disease provide a sound conceptual basis for linking the behavioral and neurological symptoms of the disease with the frequently associated pathology of amyloid plaques and neurofibrillary tangles. Out of these roots has grown the "amyloid cascade hypothesis"--a vision of the etiology of Alzheimer's that has spurred the discovery of many important insights into the neurobiology of the disease. Despite these successes, the wealth of new data now available to biomedical researchers urges a full review of the origins of Alzheimer's, and such a reconsideration is offered here. It begins with the most widely accepted risk factor for developing Alzheimer's disease: age. Then, for an individual to progress from normal age-appropriate cognitive function to a condition where the full palette of clinical symptoms is expressed, three key steps are envisioned: (1) an initiating injury, (2) a chronic neuroinflammatory response, and (3) a discontinuous cellular change of state involving most, if not all, of the cell types of the brain. The amyloid cascade is integrated into this sequence, but reconfigured as an amyloid deposition cycle. In this way, the pathology of amyloid plaques is envisioned as highly correlated with, but mechanistically distinct from, the three obligatory steps leading to Alzheimer's disease. The implications of this new model are discussed with respect to our current diagnostic criteria, and suggestions are put forward for expanding our future research efforts.
Figures

References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
-
- Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. - PubMed
-
- Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:167–179. - PubMed
-
- Arendt T, Rödel L, Gärtner U, Holzer M. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer's disease. Neuroreport. 1996;7:3047–3049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
