Projection-specific neuromodulation of medial prefrontal cortex neurons
- PMID: 21159963
- PMCID: PMC3075873
- DOI: 10.1523/JNEUROSCI.3644-10.2010
Projection-specific neuromodulation of medial prefrontal cortex neurons
Abstract
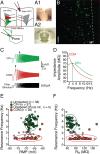
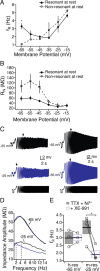
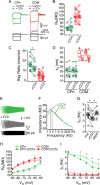
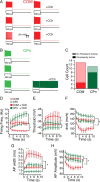
Mnemonic persistent activity in the prefrontal cortex (PFC) constitutes the neural basis of working memory. To understand how neuromodulators contribute to the generation of persistent activity, it is necessary to identify the intrinsic properties of the layer V pyramidal neurons that transfer this information to downstream networks. Here we show that the somatic dynamic and integrative properties of layer V pyramidal neurons in the rat medial PFC depend on whether they project subcortically to the pons [corticopontine (CPn)] or to the contralateral cortex [commissural (COM)]. CPn neurons display low temporal summation and accelerate in firing frequency when depolarized, whereas COM neurons have high temporal summation and display spike frequency accommodation. In response to dynamic stimuli, COM neurons act as low-pass filters, whereas CPn neurons act as bandpass filters, resonating in the theta frequency range (3-6 Hz). The disparate subthreshold properties of COM and CPn neurons can be accounted for by differences in the hyperpolarization-activated cyclic nucleotide gated cation h-current. Interestingly, neuromodulators hypothesized to enhance mnemonic persistent activity affect COM and CPn neurons distinctly. Adrenergic modulation shifts the dynamic properties of CPn but not COM neurons and increases the excitability of CPn neurons significantly more than COM neurons. In response to cholinergic modulation, CPn neurons were much more likely to display activity-dependent intrinsic persistent firing than COM neurons. Together, these data suggest that the two categories of projection neurons may subserve separate functions in PFC and may be engaged differently during working memory processes.
Figures







References
-
- Andrade R. Cell excitation enhances muscarinic cholinergic responses in rat association cortex. Brain Res. 1991;548:81–93. - PubMed
-
- Arnsten AF. Adrenergic targets for the treatment of cognitive deficits in schizophrenia. Psychopharmacology (Berl) 2004;174:25–31. - PubMed
-
- Arnsten AF. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. - PubMed
-
- Arnsten AFT. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):i6–i15. - PubMed
-
- Barth AM, Vizi ES, Zelles T, Lendvai B. 2-Adrenergic receptors modify dendritic spike generation via HCN channels in the prefrontal cortex. J Neurophysiol. 2008;99:394–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
