How, when, and where new inhibitory neurons release neurotransmitters in the adult olfactory bulb
- PMID: 21159972
- PMCID: PMC6634902
- DOI: 10.1523/JNEUROSCI.4543-10.2010
How, when, and where new inhibitory neurons release neurotransmitters in the adult olfactory bulb
Abstract
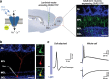
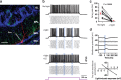
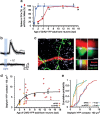
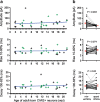
Adult-born neurons continuously incorporate into the olfactory bulb where they rapidly establish contacts with a variety of synaptic inputs. Little is known, however, about the functional properties of their output. Characterization of synaptic outputs from new neurons is essential to assess the functional impact of adult neurogenesis on mature circuits. Here, we used optogenetics to control neurotransmitter release from new neurons. We found that light-induced synaptic GABA release from adult-born neurons leads to profound modifications of postsynaptic target firing patterns. We revealed that functional output synapses form just after new cells acquire the faculty to spike, but most synapses were made a month later. Despite discrepancies in the timing of new synapse recruitment, the properties of postsynaptic signals remain constant. Remarkably, we found that all major cell types of the olfactory bulb circuit, including output neurons and several distinct subtypes of local interneurons, were contacted by adult-born neurons. Thus, this study provides new insights into how new neurons integrate into the adult neural network and may influence the sense of smell.
Figures


References
-
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. - PubMed
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Brüstle O, McKay RD. Neuronal progenitors as tools for cell replacement in the nervous system. Curr Opin Neurobiol. 1996;6:688–695. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
