More is less: a disinhibited prefrontal cortex impairs cognitive flexibility
- PMID: 21159980
- PMCID: PMC3073623
- DOI: 10.1523/JNEUROSCI.4623-10.2010
More is less: a disinhibited prefrontal cortex impairs cognitive flexibility
Abstract
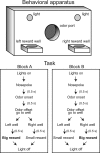
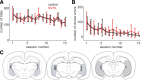
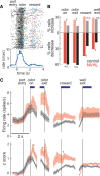
The prefrontal cortex (PFC) is critical for decision making, and it becomes dysfunctional in many neuropsychiatric disorders. Studies in schizophrenia patients and relevant animal models suggest loss of PFC inhibitory interneuron function. For instance, rats with a neonatal ventral hippocampal lesion (NVHL) show a deficient modulation of PFC interneurons by dopamine (DA). Whether the PFC becomes disinhibited in this model and alters decision making remains to be determined. Here, we recorded neural activity in the medial PFC of NVHL rats during a reward-discounting choice task that activated DA systems. Rats were trained to sample odors that instructed them to select one of two feeders that delivered unequal amounts of liquid. Putative pyramidal neurons in the PFC were hyperactive whereas task-related field potential oscillations were significantly reduced in NVHL rats, consistent with impaired interneuron activation by DA during odor sampling leading to disorganized processing. Cognitive flexibility was tested by examining response bias and errors after reversing reward outcomes. NVHL rats demonstrated impaired flexibility as they were less able to track changes in reward outcome and made more response errors than controls did. Reducing cortical excitability with the metabotropic glutamate receptor 2/3 agonist LY379268 (1 mg/kg, i.p.) improved behavioral flexibility in NVHL rats but not controls. Furthermore, D2 dopamine receptors were involved, as the antagonist eticlopride (0.02 mg/kg, i.p.) reduced the ability to switch only in control animals. We conclude that NVHL rats present PFC disinhibition, which affects neural information processing and the selection of appropriate behavioral responses.
Figures




References
-
- Arvesen JN. Jackknifing U-statistics. Ann Math Stat. 1969;40:2076–2100.
-
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. - PubMed
-
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. - PubMed
-
- Brady AM. Neonatal ventral hippocampal lesions disrupt set-shifting ability in adult rats. Behav Brain Res. 2009;205:294–298. - PubMed
-
- Cabungcal JH, Nicolas D, Kraftsik R, Cuénod M, Do KQ, Hornung JP. Glutathione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: relevance to schizophrenia. Neurobiol Dis. 2006;22:624–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
