Incomplete inactivation and rapid recovery of voltage-dependent sodium channels during high-frequency firing in cerebellar Purkinje neurons
- PMID: 21160003
- PMCID: PMC3059179
- DOI: 10.1152/jn.01056.2010
Incomplete inactivation and rapid recovery of voltage-dependent sodium channels during high-frequency firing in cerebellar Purkinje neurons
Abstract
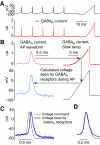
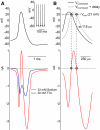
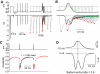
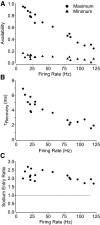
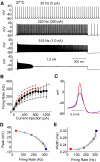
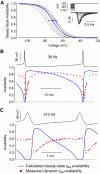
Purkinje neurons can spike very rapidly for sustained periods. We examined the cycle of sodium channel gating during high-frequency firing of Purkinje neurons, focusing on the kinetics of sodium channel inactivation and recovery during and after spikes. To analyze sodium channel availability during spiking, we recorded the firing patterns of acutely dissociated Purkinje neurons in current clamp and used these records as command voltages in voltage-clamp experiments in the same cell, adding step depolarizations at various points to assay availability. Sodium channel availability decreased abruptly during the spike, as expected, but never reached zero. During spontaneous firing (∼ 40 Hz at 37°C), availability decreased from nearly 90% before the spike to about 10-20% after the spike. With fast steady firing stimulated by current injection (∼ 300 Hz at 37°C), the availability decreased from about 60% between spikes to roughly 15-20% after the spike. Thus even at the fastest firing rates, sodium channel inactivation is incomplete after a spike, leaving a substantial fraction of sodium channels immediately available for activation. Also, inactivation recovered quickly during the early interspike interval (time constant ∼ 1 ms at 37°C), but developed slowly during the depolarization of the late interspike interval, ensuring high availability until spike threshold. These features of sodium channel gating, especially the availability remaining after the spike, reduce the refractory period and facilitate rapid repetitive firing.
Figures





References
-
- Aldrich RW, Corey DP, Stevens CF. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature 306: 436–441, 1983 - PubMed
-
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science 325: 1405–1408, 2009 - PubMed
-
- Armstrong CM. Sodium channels and gating currents. Physiol Rev 61: 644–683, 1981 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

