Encoding of eye position in the goldfish horizontal oculomotor neural integrator
- PMID: 21160010
- PMCID: PMC4103780
- DOI: 10.1152/jn.00313.2010
Encoding of eye position in the goldfish horizontal oculomotor neural integrator
Abstract
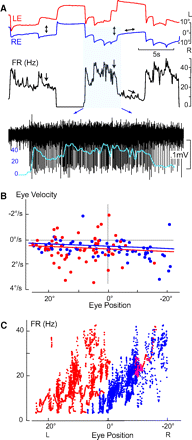
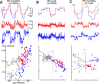
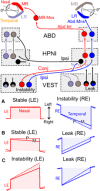
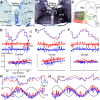
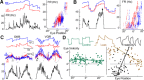
Monocular organization of the goldfish horizontal neural integrator was studied during spontaneous scanning saccadic and fixation behaviors. Analysis of neuronal firing rates revealed a population of ipsilateral (37%), conjugate (59%), and contralateral (4%) eye position neurons. When monocular optokinetic stimuli were employed to maximize disjunctive horizontal eye movements, the sampled population changed to 57, 39, and 4%. Monocular eye tracking could be elicited at different gain and phase with the integrator time constant independently modified for each eye by either centripetal (leak) or centrifugal (instability) drifting visual stimuli. Acute midline separation between the hindbrain oculomotor integrators did not affect either monocularity or time constant tuning, corroborating that left and right eye positions are independently encoded within each integrator. Together these findings suggest that the "ipsilateral" and "conjugate/contralateral" integrator neurons primarily target abducens motoneurons and internuclear neurons, respectively. The commissural pathway is proposed to select the conjugate/contralateral eye position neurons and act as a feedforward inhibition affecting null eye position, oculomotor range, and saccade pattern.
Figures
Similar articles
-
Anatomy and discharge properties of pre-motor neurons in the goldfish medulla that have eye-position signals during fixations.J Neurophysiol. 2000 Aug;84(2):1035-49. doi: 10.1152/jn.2000.84.2.1035. J Neurophysiol. 2000. PMID: 10938326
-
Dynamics of abducens nucleus neuron discharges during disjunctive saccades.J Neurophysiol. 2002 Dec;88(6):3452-68. doi: 10.1152/jn.00331.2002. J Neurophysiol. 2002. PMID: 12466460
-
Discharge dynamics of oculomotor neural integrator neurons during conjugate and disjunctive saccades and fixation.J Neurophysiol. 2003 Aug;90(2):739-54. doi: 10.1152/jn.00123.2003. Epub 2003 Apr 2. J Neurophysiol. 2003. PMID: 12672779
-
[Central oculomotor circuits].Rev Neurol (Paris). 1985;141(5):349-70. Rev Neurol (Paris). 1985. PMID: 3901182 Review. French.
-
Neural mechanisms underlying target selection with saccadic eye movements.Prog Brain Res. 2005;149:157-71. doi: 10.1016/S0079-6123(05)49012-3. Prog Brain Res. 2005. PMID: 16226583 Review.
Cited by
-
Electron Microscopic Reconstruction of Functionally Identified Cells in a Neural Integrator.Curr Biol. 2017 Jul 24;27(14):2137-2147.e3. doi: 10.1016/j.cub.2017.06.028. Epub 2017 Jul 14. Curr Biol. 2017. PMID: 28712570 Free PMC article.
-
Predicting modular functions and neural coding of behavior from a synaptic wiring diagram.Nat Neurosci. 2024 Dec;27(12):2443-2454. doi: 10.1038/s41593-024-01784-3. Epub 2024 Nov 22. Nat Neurosci. 2024. PMID: 39578573 Free PMC article.
-
Dimensionality reduction reveals separate translation and rotation populations in the zebrafish hindbrain.Curr Biol. 2023 Sep 25;33(18):3911-3925.e6. doi: 10.1016/j.cub.2023.08.037. Epub 2023 Sep 8. Curr Biol. 2023. PMID: 37689065 Free PMC article.
-
A distributed saccade-associated network encodes high velocity conjugate and monocular eye movements in the zebrafish hindbrain.Sci Rep. 2021 Jun 16;11(1):12644. doi: 10.1038/s41598-021-90315-2. Sci Rep. 2021. PMID: 34135354 Free PMC article.
-
Vestibular blueprint in early vertebrates.Front Neural Circuits. 2013 Nov 19;7:182. doi: 10.3389/fncir.2013.00182. Front Neural Circuits. 2013. PMID: 24312016 Free PMC article. Review.
References
-
- Aksay E, Baker R, Seung HS, Tank DW. Anatomy and discharge properties of pre-motor neurons in the goldfish medulla that have eye-position signals during fixations. J Neurophysiol 84: 1035–1049, 2000. - PubMed
-
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci 4: 184–193, 2001. - PubMed
-
- Aksay E, Major G, Goldman MS, Baker R, Seung HS, Tank DW. History dependence of rate covariation between neurons during persistent activity in an oculomotor integrator. Cereb Cortex 13: 1173–1184, 2003. - PubMed
-
- Allum JH, Graf W, Dichgans J, Schmidt CL. Visual-vestibular interactions in the vestibular nuclei of the goldfish. Exp Brain Res 26: 463–485, 1976. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

