Role of HIV-1 nucleocapsid protein in HIV-1 reverse transcription
- PMID: 21160280
- PMCID: PMC3073334
- DOI: 10.4161/rna.7.6.14115
Role of HIV-1 nucleocapsid protein in HIV-1 reverse transcription
Abstract
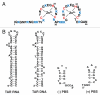
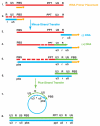
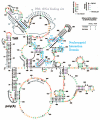
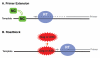
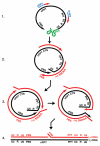
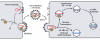
The HIV-1 nucleocapsid protein (NC) is a nucleic acid chaperone, which remodels nucleic acid structures so that the most thermodynamically stable conformations are formed. This activity is essential for virus replication and has a critical role in mediating highly specific and efficient reverse transcription. NC's function in this process depends upon three properties: (1) ability to aggregate nucleic acids; (2) moderate duplex destabilization activity; and (3) rapid on-off binding kinetics. Here, we present a detailed molecular analysis of the individual events that occur during viral DNA synthesis and show how NC's properties are important for almost every step in the pathway. Finally, we also review biological aspects of reverse transcription during infection and the interplay between NC, reverse transcriptase, and human APOBEC3G, an HIV-1 restriction factor that inhibits reverse transcription and virus replication in the absence of the HIV-1 Vif protein.
Figures
References
-
- Darlix J-L, Lapadat-Tapolsky M, de Rocquigny H, Roques BP. First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. J Mol Biol. 1995;254:523–537. - PubMed
-
- Rein A, Henderson LE, Levin JG. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem Sci. 1998;23:297–301. - PubMed
-
- Levin JG, Guo J, Rouzina I, Musier-Forsyth K. Nucleic acid chaperone activity of HIV-1 nucleocapsid protein: critical role in reverse transcription and molecular mechanism. Prog Nucleic Acid Res Mol Biol. 2005;80:217–286. - PubMed
-
- Henderson LE, Bowers MA, Sowder RC, II, Serabyn SA, Johnson DG, Bess JW, Jr, et al. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processings and complete amino acid sequences. J Virol. 1992;66:1856–1865. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
