Desmosome disassembly in response to pemphigus vulgaris IgG occurs in distinct phases and can be reversed by expression of exogenous Dsg3
- PMID: 21160493
- PMCID: PMC3235416
- DOI: 10.1038/jid.2010.389
Desmosome disassembly in response to pemphigus vulgaris IgG occurs in distinct phases and can be reversed by expression of exogenous Dsg3
Abstract
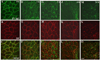
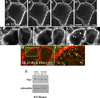
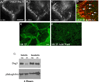
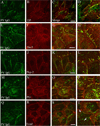
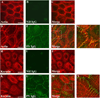
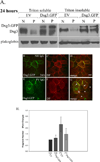
Pemphigus vulgaris (PV) is an epidermal blistering disorder caused by antibodies directed against the desmosomal cadherin desmoglein-3 (Dsg3). The mechanism by which PV IgG disrupts adhesion is not fully understood. To address this issue, primary human keratinocytes (KCs) and patient IgG were used to define the morphological, biochemical, and functional changes triggered by PV IgG. Three phases of desmosome disassembly were distinguished. Analysis of fixed and living KCs demonstrated that PV IgG cause rapid Dsg3 internalization, which likely originates from a non-junctional pool of Dsg3. Subsequently, Dsg3 and other desmosomal components rearrange into linear arrays that run perpendicular to cell contacts. Dsg3 complexes localized at the cell surface are transported in a retrograde manner along with these arrays before being released into cytoplasmic vesicular compartments. These changes in Dsg3 distribution are followed by depletion of detergent-insoluble Dsg3 pools and by the loss of cell adhesion strength. Importantly, this process of disassembly can be prevented by expressing exogenous Dsg3, thereby driving Dsg3 biosynthesis and desmosome assembly. These data support a model in which PV IgG cause the loss of cell adhesion by altering the dynamics of Dsg3 assembly into desmosomes and the turnover of cell surface pools of Dsg3 through endocytic pathways.
Conflict of interest statement
Figures


References
-
- Amagai M. Pemphigus vulgaris and its active disease mouse model. Curr Dir Autoimmun. 2008;10:167–181. - PubMed
-
- Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, et al. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem. 2005;280:23778–23784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

