Emerging roles for transthoracic ultrasonography in pulmonary diseases
- PMID: 21160632
- PMCID: PMC2999323
- DOI: 10.4329/wjr.v2.i6.203
Emerging roles for transthoracic ultrasonography in pulmonary diseases
Abstract
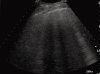
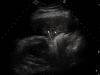
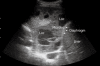
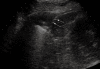
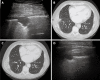
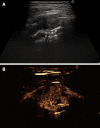
As a result of many advantages such as the absence of radiation exposure, non-invasiveness, low cost, safety, and ready availability, transthoracic ultrasonography (TUS) represents an emerging and useful technique in the management of pleural and pulmonary diseases. In this second part of a comprehensive review that deals with the role of TUS in pleuropulmonary pathology, the normal findings, sonographic artifacts and morphology of the most important and frequent pulmonary diseases are described. In particular, the usefulness of TUS in diagnosing or raising suspicion of pneumonia, pulmonary embolism, atelectasis, diffuse parenchymal diseases, adult and newborn respiratory distress syndrome, lung cancer and lung metastases are discussed, as well as its role in guidance for diagnostic and therapeutic interventional procedures. Moreover, the preliminary data about the role of contrast enhanced ultrasonography in the study of pulmonary pleural-based lesions are also reported. Finally, the limits of TUS when compared with chest computed tomography are described, highlighting the inability of TUS to depict lesions that are not in contact with the pleura or are located under bony structures, poor visualization of the mediastinum, and the need for very experienced examiners to obtain reliable results.
Keywords: Lung diseases; Pleural diseases; Ultrasonography.
Figures













References
-
- Soldati G, Copetti R. Ecografia toracica. Torino: CG Edizioni Medico-Scientifiche; 2006. pp. 12–21.
-
- Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–1646. - PubMed
-
- Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, Picano E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. 2004;93:1265–1270. - PubMed
-
- Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E. "Ultrasound comet-tail images": a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–1695. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

