Microenvironmental influences that drive progression from benign breast disease to invasive breast cancer
- PMID: 21161341
- PMCID: PMC3011086
- DOI: 10.1007/s10911-010-9195-8
Microenvironmental influences that drive progression from benign breast disease to invasive breast cancer
Abstract
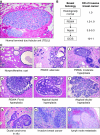
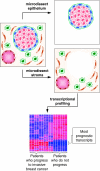
Invasive breast cancer represents the endpoint of a developmental process that originates in the terminal duct lobular units and is believed to progress through stages of increasing proliferation, atypical hyperplasia, and carcinoma in situ before the cancer acquires invasive and metastatic capabilities. By comparison with invasive breast cancer, which has been studied extensively, the preceding stages of benign breast disease are more poorly understood. Much less is known about the molecular changes underlying benign breast disease development and progression, as well as the transition from in situ into invasive disease. Even less focus has been given to the specific role of stroma in this progression. The reasons for lack of knowledge about these lesions often come from their small size and limited sample availability. More challenges are posed by limitations of the models used to investigate the lesions preceding invasive breast cancer. However, recent studies have identified alterations in stromal cell function that may be critical for disease progression from benign disease to invasive cancer: key functions of myoepithelial cells that maintain tissue structure are lost, while tissue fibroblasts become activated to produce proteases that degrade the extracellular matrix and trigger the invasive cellular phenotype. Gene expression profiling of stromal alterations associated with disease progression has also identified key transcriptional changes that occur early in disease development. In this review, we will summarize recent studies showing how stromal factors can facilitate progression of ductal carcinoma in situ to invasive disease. We also suggest approaches to identify processes that control earlier stages of disease progression.
Figures

References
-
- Arpino G, Laucirica R, Elledge RM. Premalignant and in situ breast disease: biology and clinical implications. Ann Intern Med. 2005;143(6):446–57. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

