Cell death-resistance of differentiated myotubes is associated with enhanced anti-apoptotic mechanisms compared to myoblasts
- PMID: 21161388
- PMCID: PMC3045653
- DOI: 10.1007/s10495-010-0566-9
Cell death-resistance of differentiated myotubes is associated with enhanced anti-apoptotic mechanisms compared to myoblasts
Abstract
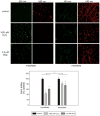
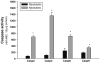
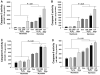
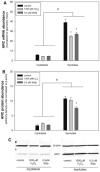
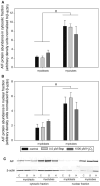
Skeletal muscle atrophy is associated with elevated apoptosis while muscle differentiation results in apoptosis resistance, indicating that the role of apoptosis in skeletal muscle is multifaceted. The objective of this study was to investigate mechanisms underlying apoptosis susceptibility in proliferating myoblasts compared to differentiated myotubes and we hypothesized that cell death-resistance in differentiated myotubes is mediated by enhanced anti-apoptotic pathways. C(2)C(12) myoblasts and myotubes were treated with H(2)O(2) or staurosporine (Stsp) to induce cell death. H(2)O(2) and Stsp induced DNA fragmentation in more than 50% of myoblasts, but in myotubes less than 10% of nuclei showed apoptotic changes. Mitochondrial membrane potential dissipation was detected with H(2)O(2) and Stsp in myoblasts, while this response was greatly diminished in myotubes. Caspase-3 activity was 10-fold higher in myotubes compared to myoblasts, and Stsp caused a significant caspase-3 induction in both. However, exposure to H(2)O(2) did not lead to caspase-3 activation in myoblasts, and only to a modest induction in myotubes. A similar response was observed for caspase-2, -8 and -9. Abundance of caspase-inhibitors (apoptosis repressor with caspase recruitment domain (ARC), and heat shock protein (HSP) 70 and -25 was significantly higher in myotubes compared to myoblasts, and in addition ARC was suppressed in response to Stsp in myotubes. Moreover, increased expression of HSPs in myoblasts attenuated cell death in response to H(2)O(2) and Stsp. Protein abundance of the pro-apoptotic protein endonuclease G (EndoG) and apoptosis-inducing factor (AIF) was higher in myotubes compared to myoblasts. These results show that resistance to apoptosis in myotubes is increased despite high levels of pro-apoptotic signaling mechanisms, and we suggest that this protective effect is mediated by enhanced anti-caspase mechanisms.
Conflict of interest statement
Figures





References
-
- Jagani Z, Khosravi-Far R. Cancer stem cells and impaired apoptosis. Adv Exp Med Biol. 2008;615:331–344. - PubMed
-
- Melet A, Song K, Bucur O, Jagani Z, Grassian AR, Khosravi-Far R. Apoptotic pathways in tumor progression and therapy. Adv Exp Med Biol. 2008;615:47–79. - PubMed
-
- Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. - PubMed
-
- Kermer P, Liman J, Weishaupt JH, Bahr M. Neuronal apoptosis in neurodegenerative diseases: from basic research to clinical application. Neurodegener Dis. 2004;1:9–19. - PubMed
-
- Adams V, Jiang H, Yu J, et al. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999;33:959–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

