Formulation and in vitro evaluation of xanthan gum or carbopol 934-based mucoadhesive patches, loaded with nicotine
- PMID: 21161460
- PMCID: PMC3066338
- DOI: 10.1208/s12249-010-9534-5
Formulation and in vitro evaluation of xanthan gum or carbopol 934-based mucoadhesive patches, loaded with nicotine
Abstract
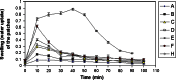
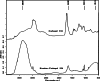
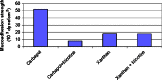
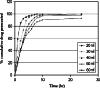
Bilayer nicotine mucoadhesive patches were prepared and evaluated to determine the feasibility of the formulation as a nicotine replacement product to aid in smoking cessation. Nicotine patches were prepared using xanthan gum or carbopol 934 as a mucoadhesive polymers and ethyl cellulose as a backing layer. The patches were evaluated for their thickness, weight and content uniformity, swelling behavior, drug-polymers interaction, adhesive properties, and drug release. The physicochemical interactions between nicotine and the polymers were investigated by Fourier transform infrared (FTIR) spectroscopy. Mucoadhesion was assessed using two-arm balance method, and the in vitro release was studied using the Franz cell. FTIR revealed that there was an acid base interaction between nicotine and carbopol as well as nicotine and xanthan. Interestingly, the mucoadhesion and in vitro release studies indicated that this interaction was strong between the drug and carbopol whereas it was weak between the drug and xanthan. Loading nicotine concentration to non-medicated patches showed a significant decrease in the mucoadhesion strength of carbopol patches and no significant effect on the mucoadhesion strength of xanthan patches. In vitro release studies of the xanthan patches showed a reasonable fast initial release profile followed by controlled drug release over a 10-h period.
© 2010 American Association of Pharmaceutical Scientists
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

