Clinical efficacy and safety results for dose escalation of somatostatin receptor ligands in patients with acromegaly: a literature review
- PMID: 21161602
- PMCID: PMC3094533
- DOI: 10.1007/s11102-010-0282-z
Clinical efficacy and safety results for dose escalation of somatostatin receptor ligands in patients with acromegaly: a literature review
Abstract
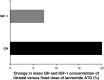
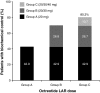
Acromegaly is a rare disease with a multifaceted clinical presentation. In 90-95% of patients with acromegaly, the disease is caused by a growth hormone (GH)-secreting pituitary adenoma with elevated GH levels that ultimately induce excessive hepatic secretion of insulin-like growth factor-1 (IGF-1). Somatostatin receptor ligands (SRLs) are considered the standard medical choice for the treatment of acromegaly, and normalization of GH and IGF-1 is attainable with effective therapy. This review aims to summarize the literature relative to SRL dose escalation therapy in patients with acromegaly. A United States National Library of Medicine PubMed search of SRL's was conducted using the following search terms: ((((LAR) OR ATG) OR octreotide) OR lanreotide Autogel) AND acromegaly. Related articles in non peer-reviewed journals were excluded. The rationale and benefits of SRL dose optimization therapy were investigated with emphasis on describing the clinical recognition, treatment, and management of patients with acromegaly. We found that dose escalation could provide additional biochemical control of acromegaly in patients who are inadequately controlled with conventional starting doses of octreotide LAR and lanreotide Autogel(®). Furthermore, patients should routinely have their GH and IGF-1 levels closely monitored and their SRL dose increased or decreased thereafter according to individual response.
© The Author(s) 2010. This article is published with open access at Springerlink.com
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

