Network analysis of associations between serum interferon-α activity, autoantibodies, and clinical features in systemic lupus erythematosus
- PMID: 21162028
- PMCID: PMC3068224
- DOI: 10.1002/art.30187
Network analysis of associations between serum interferon-α activity, autoantibodies, and clinical features in systemic lupus erythematosus
Abstract
Objective: Interferon-α (IFNα) is a primary pathogenic factor in systemic lupus erythematosus (SLE), and high IFNα levels may be associated with particular clinical manifestations. The prevalence of individual clinical and serologic features differs significantly by ancestry. This study was undertaken to detect associations between clinical and serologic disease manifestations and serum IFNα activity in a large diverse SLE cohort, using multivariate and network analyses.
Methods: We studied 1,089 SLE patients (387 African American, 186 Hispanic American, and 516 European American patients). The presence or absence of individual American College of Rheumatology (ACR) clinical criteria for SLE, autoantibodies, and serum IFNα activity data were analyzed in univariate and multivariate models. Iterative multivariate logistic regression was performed in each ancestral background group separately to establish the network of associations between variables that were independently significant following Bonferroni correction.
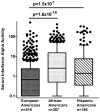
Results: In all ancestral backgrounds, high IFNα activity was associated with anti-Ro and anti-double-stranded DNA antibodies (P = 4.6 × 10(-18) and P = 2.9 × 10(-16) , respectively). Younger age, non-European ancestry, and anti-RNP were also independently associated with increased serum IFNα activity (P ≤ 6.7 × 10(-4) ). We found 14 unique associations between variables in network analysis, and only 7 of these associations were shared among >1 ancestral background. Associations between clinical criteria were different for different ancestral backgrounds, while autoantibody-IFNα relationships were similar across backgrounds. IFNα activity and autoantibodies were not associated with ACR clinical features in multivariate models.
Conclusion: Our findings indicate that serum IFNα activity is strongly and consistently associated with autoantibodies, and not independently associated with clinical features in SLE. IFNα may be more relevant to humoral tolerance and initial pathogenesis than later clinical disease manifestations.
Copyright © 2011 by the American College of Rheumatology.
Conflict of interest statement
Figures

References
-
- Ronnblom LE, Alm GV, Oberg KE. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med. 1990;227(3):207–10. - PubMed
-
- Niewold TB, Swedler WI. Systemic lupus erythematosus arising during interferon-alpha therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin Rheumatol. 2005;24(2):178–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE015223/DE/NIDCR NIH HHS/United States
- AI-83194/AI/NIAID NIH HHS/United States
- K08-AI-083790/AI/NIAID NIH HHS/United States
- R01 AI024717/AI/NIAID NIH HHS/United States
- RR-15577/RR/NCRR NIH HHS/United States
- P50 AR048940/AR/NIAMS NIH HHS/United States
- RR-20143/RR/NCRR NIH HHS/United States
- AR-058554/AR/NIAMS NIH HHS/United States
- AR-053483/AR/NIAMS NIH HHS/United States
- P01 AI083194/AI/NIAID NIH HHS/United States
- R21 AI053747/AI/NIAID NIH HHS/United States
- AR-62277/AR/NIAMS NIH HHS/United States
- AI-62629/AI/NIAID NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- P20 RR020143/RR/NCRR NIH HHS/United States
- U19 AI082714/AI/NIAID NIH HHS/United States
- P30 GM103510/GM/NIGMS NIH HHS/United States
- AI-31584/AI/NIAID NIH HHS/United States
- AR-49084/AR/NIAMS NIH HHS/United States
- UL1 RR024999/RR/NCRR NIH HHS/United States
- AR-045084/AR/NIAMS NIH HHS/United States
- RC1 AR058554/AR/NIAMS NIH HHS/United States
- P30 AR053483/AR/NIAMS NIH HHS/United States
- R01 AI031584/AI/NIAID NIH HHS/United States
- DE-15223/DE/NIDCR NIH HHS/United States
- K08 AI083790/AI/NIAID NIH HHS/United States
- P20 RR015577/RR/NCRR NIH HHS/United States
- AR-42460/AR/NIAMS NIH HHS/United States
- UL1-RR-024999/RR/NCRR NIH HHS/United States
- P30 RR031152/RR/NCRR NIH HHS/United States
- N01 AR062277/AR/NIAMS NIH HHS/United States
- AI-082714/AI/NIAID NIH HHS/United States
- AI-071651/AI/NIAID NIH HHS/United States
- AI-24717/AI/NIAID NIH HHS/United States
- AR-48940/AR/NIAMS NIH HHS/United States
- U19 AI062629/AI/NIAID NIH HHS/United States
- R37 AI024717/AI/NIAID NIH HHS/United States
- R01 AR042460/AR/NIAMS NIH HHS/United States
- L30 AI071651/AI/NIAID NIH HHS/United States
- AI-53747/AI/NIAID NIH HHS/United States
- P30-DK-42086/DK/NIDDK NIH HHS/United States
- P01 AR049084/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

