Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro
- PMID: 21163906
- PMCID: PMC3044205
- DOI: 10.1093/toxsci/kfq379
Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro
Abstract
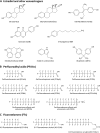
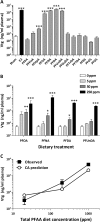
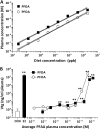
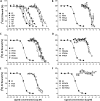
The objectives of this study were to determine the structural characteristics of perfluoroalkyl acids (PFAAs) that confer estrogen-like activity in vivo using juvenile rainbow trout (Oncorhynchus mykiss) as an animal model and to determine whether these chemicals interact directly with the estrogen receptor (ER) using in vitro and in silico species comparison approaches. Perfluorooctanoic (PFOA), perfluorononanoic (PFNA), perfluorodecanoic (PFDA), and perfluoroundecanoic (PFUnDA) acids were all potent inducers of the estrogen-responsive biomarker protein vitellogenin (Vtg) in vivo, although at fairly high dietary exposures. A structure-activity relationship for PFAAs was observed, where eight to ten fluorinated carbons and a carboxylic acid end group were optimal for maximal Vtg induction. These in vivo findings were corroborated by in vitro mechanistic assays for trout and human ER. All PFAAs tested weakly bound to trout liver ER with half maximal inhibitory concentration (IC(50)) values of 15.2-289 μM. Additionally, PFOA, PFNA, PFDA, PFUnDA, and perlfuorooctane sulfonate (PFOS) significantly enhanced human ERα-dependent transcriptional activation at concentrations ranging from 10-1000 nM. Finally, we employed an in silico computational model based upon the crystal structure for the human ERα ligand-binding domain complexed with E2 to structurally investigate binding of these putative ligands to human, mouse, and trout ERα. PFOA, PFNA, PFDA, and PFOS all efficiently docked with ERα from different species and formed a hydrogen bond at residue Arg394/398/407 (human/mouse/trout) in a manner similar to the environmental estrogens bisphenol A and nonylphenol. Overall, these data support the contention that several PFAAs are weak environmental xenoestrogens of potential concern.
Figures

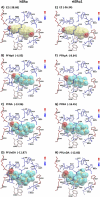
References
-
- Abagyan R, Totrov M, Kuznetsov D. ICM—a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J. Comput. Chem. 1994;15:488–506.
-
- Abdellatif AG, Preat V, Taper HS, Roberfroid M. The modulation of rat liver carcinogenesis by perfluorooctanoic acid, a peroxisome proliferator. Toxicol. Appl. Pharmacol. 1991;111:530–537. - PubMed
-
- Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol. Sci. 2000;54:138–153. - PubMed

