Neoangiogenesis contributes to the development of hemophilic synovitis
- PMID: 21163925
- PMCID: PMC3317791
- DOI: 10.1182/blood-2010-05-284653
Neoangiogenesis contributes to the development of hemophilic synovitis
Abstract
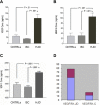
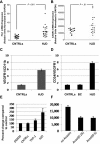
Joint arthropathy secondary to recurrent hemarthroses remains a debilitating complication of hemophilia despite the use of prophylactic factor concentrates. Increased vascularity and neoangiogenesis have been implicated in the progression of musculoskeletal disorders and tumor growth. We hypothesized that de novo blood vessel formation could play a major role in the pathogenesis of hemophilic joint disease (HJD). We observed a 4-fold elevation in proangiogenic factors (vascular endothelial growth factor-A [VEGF-A], stromal cell-derived factor-1, and matrix metalloprotease-9) and proangiogenic macrophage/monocyte cells (VEGF(+)/CD68(+) and VEGFR1(+)/CD11b(+)) in the synovium and peripheral blood of HJD subjects along with significantly increased numbers of VEGFR2(+)/AC133(+) endothelial progenitor cells and CD34(+)/VEGFR1(+) hematopoietic progenitor cells. Sera from HJD subjects induced an angiogenic response in endothelial cells that was abrogated by blocking VEGF, whereas peripheral blood mononuclear cells from HJD subjects stimulated synovial cell proliferation, which was blocked by a humanized anti-VEGF antibody (bevacizumab). Human synovial cells, when incubated with HJD sera, could elicit up-regulation of HIF-1α mRNA with HIF-1α expression in the synovium of HJD subjects, implicating hypoxia in the neoangiogenesis process. Our results provide evidence of local and systemic angiogenic response in hemophilic subjects with recurrent hemarthroses suggesting a potential to develop surrogate biologic markers to identify the onset and progression of hemophilic synovitis.
Figures




Comment in
-
Unraveling hemophilic arthropathy.Blood. 2011 Feb 24;117(8):2302-3. doi: 10.1182/blood-2011-01-327312. Blood. 2011. PMID: 21350060
References
-
- Manco-Johnson MJ, Riske B, Casper CK. Advances in care of children with hemophilia. Semin Thromb Haemost. 2003;29(6):585–594. - PubMed
-
- Rodriguez-Merchan EC. Pathogenesis, early diagnosis and prophylaxis for chronic hemophilic synovitis. Clin Orthop. 1997;343:6–11. - PubMed
-
- Hoots WK. Pathogenesis of hemophilia arthropathy. Semin Hematol. 2006;43(suppl 1):S18–S22. - PubMed
-
- Madhok R, Bennett D, Sturrock RD, Forbes CD. Mechanisms of joint damage in an experimental model of hemophilic arthritis. Arthritis Rheum. 1988;31(9):1148–1155. - PubMed
-
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

