Plasticity in membrane cholesterol contributes toward electrical maturation of hearing
- PMID: 21163952
- PMCID: PMC3037689
- DOI: 10.1074/jbc.M110.186486
Plasticity in membrane cholesterol contributes toward electrical maturation of hearing
Abstract
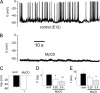
Advances in refining the "fluid mosaic" model of the plasma membrane have revealed that it is wrought with an ordered lipid composition that undergoes remarkable plasticity during cell development. Despite the evidence that specific signaling proteins and ion channels gravitate toward these lipid microdomains, identification of their functional impact remains a formidable challenge. We report that in contrast to matured auditory hair cells, depletion of membrane cholesterol in developing hair cells produced marked potentiation of voltage-gated K(+) currents (I(Kv)). The enhanced magnitude of I(Kv) in developing hair cells was in keeping with the reduced cholesterol-rich microdomains in matured hair cells. Remarkably, potentiation of the cholesterol-sensitive current was sufficient to abolish spontaneous activity, a functional blueprint of developing and regenerating hair cells. Collectively, these findings provide evidence that developmental plasticity of lipid microdomains and the ensuing changes in K(+) currents are important determinants of one of the hallmarks in the maturation of hearing.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

