Cholinergic interneurons control local circuit activity and cocaine conditioning
- PMID: 21164015
- PMCID: PMC3142356
- DOI: 10.1126/science.1193771
Cholinergic interneurons control local circuit activity and cocaine conditioning
Abstract
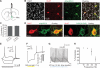
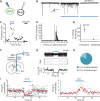
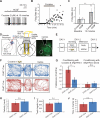
Cholinergic neurons are widespread, and pharmacological modulation of acetylcholine receptors affects numerous brain processes, but such modulation entails side effects due to limitations in specificity for receptor type and target cell. As a result, causal roles of cholinergic neurons in circuits have been unclear. We integrated optogenetics, freely moving mammalian behavior, in vivo electrophysiology, and slice physiology to probe the cholinergic interneurons of the nucleus accumbens by direct excitation or inhibition. Despite representing less than 1% of local neurons, these cholinergic cells have dominant control roles, exerting powerful modulation of circuit activity. Furthermore, these neurons could be activated by cocaine, and silencing this drug-induced activity during cocaine exposure (despite the fact that the manipulation of the cholinergic interneurons was not aversive by itself) blocked cocaine conditioning in freely moving mammals.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

