The regulation of parathyroid hormone secretion and synthesis
- PMID: 21164021
- PMCID: PMC5546216
- DOI: 10.1681/ASN.2010020186
The regulation of parathyroid hormone secretion and synthesis
Abstract
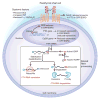
Secondary hyperparathyroidism classically appears during the course of chronic renal failure and sometimes after renal transplantation. Understanding the mechanisms by which parathyroid hormone (PTH) synthesis and secretion are normally regulated is important in devising methods to regulate overactivity and hyperplasia of the parathyroid gland after the onset of renal insufficiency. Rapid regulation of PTH secretion in response to variations in serum calcium is mediated by G-protein coupled, calcium-sensing receptors on parathyroid cells, whereas alterations in the stability of mRNA-encoding PTH by mRNA-binding proteins occur in response to prolonged changes in serum calcium. Independent of changes in intestinal calcium absorption and serum calcium, 1α,25-dihydroxyvitamin D also represses the transcription of PTH by associating with the vitamin D receptor, which heterodimerizes with retinoic acid X receptors to bind vitamin D-response elements within the PTH gene. 1α,25-Dihydroxyvitamin D additionally regulates the expression of calcium-sensing receptors to indirectly alter PTH secretion. In 2°HPT seen in renal failure, reduced concentrations of calcium-sensing and vitamin D receptors, and altered mRNA-binding protein activities within the parathyroid cell, increase PTH secretion in addition to the more widely recognized changes in serum calcium, phosphorus, and 1α,25-dihydroxyvitamin D. The treatment of secondary hyperparathyroidism by correction of serum calcium and phosphorus concentrations and the administration of vitamin D analogs and calcimimetic agents may be augmented in the future by agents that alter the stability of mRNA-encoding PTH.
Figures
References
-
- Habener JF, Rosenblatt M, Potts JT., Jr Parathyroid hormone: Biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev. 1984;64:985–1053. - PubMed
-
- Potts JT. Parathyroid hormone: Past and present. J Endocrinol. 2005;187:311–325. - PubMed
-
- Kumar R. Metabolism of 1,25-dihydroxyvitamin D3. Physiol Rev. 1984;64:478–504. - PubMed
-
- DeLuca HF, Schnoes HK. Vitamin D: Recent advances. Annu Rev Biochem. 1983;52:411–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

