Autoantibodies against cell surface GRP78 promote tumor growth in a murine model of melanoma
- PMID: 21164368
- PMCID: PMC3116076
- DOI: 10.1097/CMR.0b013e3283426805
Autoantibodies against cell surface GRP78 promote tumor growth in a murine model of melanoma
Abstract
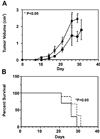
Autoantibodies that react with GRP78 expressed on the cell-surface of many tumor cell lines occur in the sera of patients with prostate cancer, melanoma, and ovarian cancer. These autoantibodies are a negative prognostic factor in prostate cancer and, when purified, stimulate tumor cell proliferation in vitro. It is unclear, however, whether these immunoglobulin Gs are merely a biomarker, or whether they actually promote the tumor growth in vivo. We immunized C57Bl/6 mice with recombinant GRP78 and then implanted the B16F1 murine melanoma cell line as flank tumors. We used the antisera from these mice for in-vitro cell signaling and proliferation assays. The immunodominant epitope in patients with cancer was well represented in the antibody repertoire of these immunized mice. We observed significantly accelerated tumor growth, and shortened survival in GRP78-immunized mice compared with controls. Furthermore, antisera from these mice, and purified anti-GRP78 immunoglobulin G from similarly immunized mice, stimulate Akt phosphorylation and proliferation in B16F1 and human DM6 melanoma cells in culture. These studies show a causal link between a humoral response to GRP78 and the progression of cancer in a murine melanoma model. They support the hypothesis that such autoantibodies are involved in the progression of human cancers and are not simply a biomarker. As GRP78 is present on the surface of many types of cancer cells, this hypothesis has broad clinical and therapeutic implications.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures





Similar articles
-
Changes in oligosaccharide chains of autoantibodies to GRP78 expressed during progression of malignant melanoma stimulate melanoma cell growth and survival.Melanoma Res. 2011 Aug;21(4):323-34. doi: 10.1097/CMR.0b013e3283471042. Melanoma Res. 2011. PMID: 21597391 Free PMC article.
-
Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum.Cancer Res. 2006 Dec 1;66(23):11424-31. doi: 10.1158/0008-5472.CAN-06-1721. Cancer Res. 2006. PMID: 17145889
-
A murine monoclonal antibody directed against the carboxyl-terminal domain of GRP78 suppresses melanoma growth in mice.Melanoma Res. 2012 Jun;22(3):225-35. doi: 10.1097/CMR.0b013e32835312fd. Melanoma Res. 2012. PMID: 22495669
-
GRP78 signaling hub a receptor for targeted tumor therapy.Adv Genet. 2010;69:97-114. doi: 10.1016/S0065-2660(10)69006-2. Adv Genet. 2010. PMID: 20807604 Review.
-
Glucose-regulated protein (GRP78) is an important cell surface receptor for viral invasion, cancers, and neurological disorders.IUBMB Life. 2021 Jun;73(6):843-854. doi: 10.1002/iub.2502. Epub 2021 May 15. IUBMB Life. 2021. PMID: 33960608 Review.
Cited by
-
GRP78 Protein Expression in Ovarian Cancer Patients and Perspectives for a Drug-Targeting Approach.J Oncol. 2012;2012:468615. doi: 10.1155/2012/468615. Epub 2012 Mar 18. J Oncol. 2012. PMID: 22481929 Free PMC article.
-
Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential.Nat Rev Cancer. 2014 Apr;14(4):263-76. doi: 10.1038/nrc3701. Nat Rev Cancer. 2014. PMID: 24658275 Free PMC article. Review.
-
Activated α2-macroglobulin binding to human prostate cancer cells triggers insulin-like responses.J Biol Chem. 2015 Apr 10;290(15):9571-87. doi: 10.1074/jbc.M114.617837. Epub 2015 Feb 26. J Biol Chem. 2015. PMID: 25720493 Free PMC article.
-
Role of the unfolded protein response in determining the fate of tumor cells and the promise of multi-targeted therapies.Cell Stress Chaperones. 2018 May;23(3):317-334. doi: 10.1007/s12192-017-0844-3. Epub 2017 Sep 27. Cell Stress Chaperones. 2018. PMID: 28952072 Free PMC article. Review.
-
Autoantibodies against the cell surface-associated chaperone GRP78 stimulate tumor growth via tissue factor.J Biol Chem. 2017 Dec 22;292(51):21180-21192. doi: 10.1074/jbc.M117.799908. Epub 2017 Oct 24. J Biol Chem. 2017. PMID: 29066620 Free PMC article.
References
-
- Quinones QJ, de Ridder GG, Pizzo SV. GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol Histopathol. 2008;23:1409–1416. - PubMed
-
- Cai B, Tomida A, Mikami K, Nagata K, Tsuruo T. Down-regulation of epidermal growth factor receptor-signaling pathway by binding of GRP78/BiP to the receptor under glucose-starved stress conditions. J Cell Physiol. 1998;177:282–288. - PubMed
-
- Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. - PubMed
-
- Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. - PubMed
-
- Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

