Manufacturing and in vivo inner ear visualization of MRI traceable liposome nanoparticles encapsulating gadolinium
- PMID: 21167059
- PMCID: PMC3016339
- DOI: 10.1186/1477-3155-8-32
Manufacturing and in vivo inner ear visualization of MRI traceable liposome nanoparticles encapsulating gadolinium
Abstract
Background: Treatment of inner ear diseases remains a problem because of limited passage through the blood-inner ear barriers and lack of control with the delivery of treatment agents by intravenous or oral administration. As a minimally-invasive approach, intratympanic delivery of multifunctional nanoparticles (MFNPs) carrying genes or drugs to the inner ear is a future therapy for treating inner ear diseases, including sensorineural hearing loss (SNHL) and Meniere's disease. In an attempt to track the dynamics and distribution of nanoparticles in vivo, here we describe manufacturing MRI traceable liposome nanoparticles by encapsulating gadolinium-tetra-azacyclo-dodecane-tetra-acetic acid (Gd-DOTA) (abbreviated as LPS+Gd-DOTA) and their distribution in the inner ear after either intratympanic or intracochlear administration.
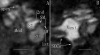
Results: Measurements of relaxivities (r1 and r2) showed that LPS+Gd-DOTA had efficient visible signal characteristics for MRI. In vivo studies demonstrated that LPS+Gd-DOTA with 130 nm size were efficiently taken up by the inner ear at 3 h after transtympanic injection and disappeared after 24 h. With intracochlear injection, LPS+Gd-DOTA were visualized to distribute throughout the inner ear, including the cochlea and vestibule with fast dynamics depending on the status of the perilymph circulation.
Conclusion: Novel LPS+Gd-DOTA were visible by MRI in the inner ear in vivo demonstrating transport from the middle ear to the inner ear and with dynamics that correlated to the status of the perilymph circulation.
Figures










References
-
- Nanoear: 3g-Nanotechnology based targeted drug delivery using the inner ear as a model target organ. http://www.nanoear.org/
-
- Zou J, Saulnier P, Perrier T, Zhang Y, Manninen T, Toppila E, Pyykko I. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J Biomed Mater Res B Appl Biomater. 2008;87:10–18. - PubMed
-
- Zou J, Zhang Y, Zhang W, Ranjan S, Sood R, Mikhailov A, Kinnunen P, Pyykko I. Internalization of liposome nanoparticles functionalized with TrkB ligand in rat cochlear cell populations. European Journal of Nenomedicine. 2009;3:8–14.
LinkOut - more resources
Full Text Sources
Research Materials

