IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling
- PMID: 21167580
- PMCID: PMC3049834
- DOI: 10.1016/j.jaci.2010.11.009
IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling
Abstract
Background: In atopic subjects food ingestion drives the production of IgE antibodies that can trigger hypersensitivity reactions. The IL-4 pathway plays a critical role in this response, and genetic polymorphisms in its components have been linked to allergy.
Objective: We sought to test whether an activating mutation in the IL-4 receptor (IL-4R) α chain enhances allergic responses to a food antigen.
Methods: F709 mice, in which the IL-4Rα immunoreceptor tyrosine-based inhibitory motif is inactivated, were gavage fed with ovalbumin (OVA). Reactions to OVA challenge and immune responses, including antibody production and T(H)2 responses, were assessed.
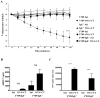
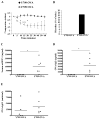
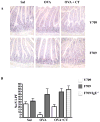
Results: F709 mice, but not wild-type control animals, sensitized by means of gavage with OVA and either cholera toxin or staphylococcal enterotoxin B, displayed mast cell activation and systemic anaphylaxis on enteral challenge. Anaphylaxis was elicited even in F709 mice enterally sensitized with OVA alone. Bone marrow chimera experiments established that the increased sensitivity conferred by the F709 genotype was mediated mostly by hematopoietic cells but that nonhematopoietic cells also contributed. F709 mice exhibited increased intestinal permeability to macromolecules. The F709 genotype conferred increased OVA-specific IgE but not IgG1 responses, local and systemic T(H)2 responses, and intestinal mast cell hyperplasia compared with wild-type mice. Anaphylaxis was abrogated in F709 mice lacking IgE or the high-affinity receptor for IgE (FcεRI).
Conclusion: Augmented IL-4Rα signaling confers increased intestinal permeability and dramatically enhanced sensitivity to food allergens. Unlike anaphylaxis to injected antigens, which in rodents can be mediated by either IgE or IgG antibodies, the food-induced response in F709 mice is solely IgE dependent.
Copyright © 2010 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Figures




References
-
- Eigenmann PA, Beyer K, Wesley Burks A, Lack G, Liacouras CA, Hourihane JO, et al. New visions for food allergy: an iPAC summary and future trends. Pediatr Allergy Immunol. 2008;19 (Suppl 19):26–39. - PubMed
-
- Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006;117:S470–5. - PubMed
-
- Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009;60:261–77. - PubMed
-
- Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10:493–9. - PubMed
-
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI054471/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 AI007512/AI/NIAID NIH HHS/United States
- R21 AI080002/AI/NIAID NIH HHS/United States
- 1R21AI087666/AI/NIAID NIH HHS/United States
- R01 AI083516/AI/NIAID NIH HHS/United States
- R21 AI087666/AI/NIAID NIH HHS/United States
- 5T32AI007512-24/AI/NIAID NIH HHS/United States
- R01 AI065617/AI/NIAID NIH HHS/United States
- 2R01AI065617/AI/NIAID NIH HHS/United States
- R01-AI054471/AI/NIAID NIH HHS/United States
- 1R21AI080002/AI/NIAID NIH HHS/United States
- AI083516/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

