Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin
- PMID: 21169216
- PMCID: PMC3017160
- DOI: 10.1073/pnas.1016785108
Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin
Abstract
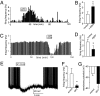
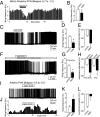
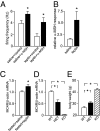
Melanocortin-4 receptor (MC4R) is critical for energy homeostasis, and the paraventricular nucleus of the hypothalamus (PVN) is a key site of MC4R action. Most studies suggest that leptin regulates PVN neurons indirectly, by binding to receptors in the arcuate nucleus or ventromedial hypothalamus and regulating release of products like α-melanocyte-stimulating hormone (α-MSH), neuropeptide Y (NPY), glutamate, and GABA from first-order neurons onto the MC4R PVN cells. Here, we investigate mechanisms underlying regulation of activity of these neurons under various metabolic states by using hypothalamic slices from a transgenic MC4R-GFP mouse to record directly from MC4R neurons. First, we show that in vivo leptin levels regulate the tonic firing rate of second-order MC4R PVN neurons, with fasting increasing firing frequency in a leptin-dependent manner. We also show that, although leptin inhibits these neurons directly at the postsynaptic membrane, α-MSH and NPY potently stimulate and inhibit the cells, respectively. Thus, in contrast with the conventional model of leptin action, the primary control of MC4R PVN neurons is unlikely to be mediated by leptin action on arcuate NPY/agouti-related protein and proopiomelanocortin neurons. We also show that the activity of MC4R PVN neurons is controlled by the constitutive activity of the MC4R and that expression of the receptor mRNA and α-MSH sensitivity are both stimulated by leptin. Thus, leptin acts multinodally on arcuate nucleus/PVN circuits to regulate energy homeostasis, with prominent mechanisms involving direct control of both membrane conductances and gene expression in the MC4R PVN neuron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
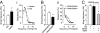
References
-
- Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. - PubMed
-
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. - PubMed
-
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. - PubMed
-
- Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. - PubMed
-
- Fekete C, et al. α-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000;20:1550–1558. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

