High-throughput plasmid content analysis of Borrelia burgdorferi B31 by using Luminex multiplex technology
- PMID: 21169439
- PMCID: PMC3067200
- DOI: 10.1128/AEM.01877-10
High-throughput plasmid content analysis of Borrelia burgdorferi B31 by using Luminex multiplex technology
Abstract
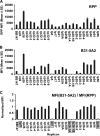
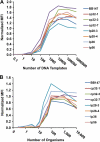
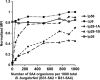
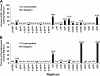
Borrelia burgdorferi, the causative agent of Lyme disease in North America, is an invasive pathogen that causes persistent multiorgan manifestations in humans and other mammals. Genetic studies of this bacterium are complicated by the presence of multiple plasmid replicons, many of which are readily lost during in vitro culture. The analysis of B. burgdorferi plasmid content by plasmid-specific PCR and agarose gel electrophoresis or other existing techniques is informative, but these techniques are cumbersome and challenging to perform in a high-throughput manner. In this study, a PCR-based Luminex assay was developed for determination of the plasmid content of the strain B. burgdorferi B31. This multiplex, high-throughput method allows simultaneous detection of the plasmid contents of many B. burgdorferi strains in a 96-well format. The procedure was used to evaluate the occurrence of plasmid loss in 44 low-passage B. burgdorferi B31 clones and in a library of over 4,000 signature-tagged mutagenesis (STM) transposon mutant clones. This analysis indicated that only 40% of the clones contained all plasmids, with (in order of decreasing frequency) lp5, lp56, lp28-1, lp25, cp9, lp28-4, lp28-2, and lp21 being the most commonly missing plasmids. These results further emphasize the need for careful plasmid analysis in Lyme disease Borrelia studies. Adaptations of this approach may also be useful in the evaluation of plasmid content and chromosomal gene variations in additional Lyme disease Borrelia strains and other organisms with variable genomes and in the correlation of these genetic differences with pathogenesis and other biological properties.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

